What Is Strontium Titanate Used For?
Strontium titanate is a compound formed by the burning of hydrogen with oxygen under certain conditions. The process of forming strontium titanate requires an excess amount of oxygen because the titanium component does not fully oxidize without the oxygen. In order to form this chemical, 1.5 volumes of hydrogen must be mixed with the feed powder. The titanium tetrachloride is a highly pure compound.
The mineral was discovered in 1982 and subsequently patented. Later, it was found in Japan and Paraguay. It is a rare and expensive mineral that is present only in small crystals. It is so rare that it has no commercial use. Although it is an excellent simulant for diamond, there is one major problem. This substance is much harder to work with than diamond. The material is very brittle and difficult to work with, making it unsuitable for many applications.
The main drawback of this material is that it is harder than diamond, making it difficult to work with. The best method is to use it in a liquid, such as acetonitrile. Using an acid such as acetonitrile will result in a lower solubility level. When mixed with a solvent, it will form an oxide sol. This is not the best option for chemical production.
The synthetic form of strontium titanate was discovered in 1982, but it wasn't widely available until the 1980s. NL Industries began researching the material. Merker and Lynd, two scientists at the National Lead Company, patented a method for producing uniform spherical particles of the element. They also added colouring dopants to the feed powder for a better visual appearance.
Strontium titanate is a man-made substance with a chemical composition of SrTiO 3. It was initially discovered as a synthetic diamond simulant in the early 1950s and gained attention as a result. However, despite its chemical similarities to diamond, it is not as hard as diamond, and so its value depends on its use. The most common uses of strontium titanate include:
The chemical composition of strontium titanate is SrTiO3. This substance is a man-made chemical. Its structure and composition are different from diamond. It is used as a replacement for diamond. When it is combined with other materials, it can make a diamond more durable and stronger. The same effect can be achieved with this metal. It is often employed in electronics. So what is strontium titanate?
While strontium titanate is a synthetic material, it can be used in many ways. This material is commonly known as sputtering targets. It is also a source of calcium. It can be manufactured as a chemical compound. There are several different uses for this metal. Its use is varied. There are various types of titanium products. The primary application for this alloy is in manufacturing.
Strontium titanate is an alloy of strontium and titanium. It is a centrosymmetric ferroelectric material. It was thought to be a synthetic compound until it was discovered in Siberia in 1982. Until that time, it was thought to be an artificial mineral until its discovery. Until the late 1980s, however, it was found to be naturally occurring. It is used in precision optics, advanced ceramics, and other applications.
The mineral is used to make cathode ray tubes in televisions. It is also used in certain cancer treatments. It is found in the minerals celestite and strontianite. It was named after the town of its discovery in Scotland. The metal is found in numerous forms in nature. It is important for the development of electronic components. When the alloy is manufactured, it is referred to as a high-grade titanium alloy.
Strontium titanate is a high-grade material with similar characteristics to diamond. It has high dielectric constant and high luster. It is an excellent material for capacitors. Its ability to conduct electricity makes it a desirable material for solar cells. While strontium titanate is cheaper than diamond, its durability makes it an attractive alternative for many consumers. The price is significantly lower than the price of diamond.
SrTiO3 Crystal Structure
The SrTiO3 crystal structure is quite similar to other silicon materials in terms of its structure, which is largely determined by the band gap and the electron distribution. This is mainly due to the presence of two different types of electrons - the Ce and the Pr - in SrTiO3. However, the difference in the energies of the two electrons makes it more difficult to determine which one is dominant in the material.
During the formation of the SrTiO3 crystal structure, the presence of RE atoms (the element that does not 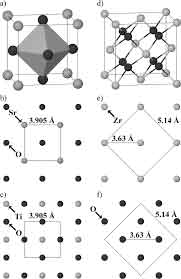 have an electron) is a sign of a mono-doped structure. This means that the Sr, Ti, and O atoms are homogenous and mono-doped in the material. The energies of the two atomic species are measured from bulk Sr and Ti crystal structures.
have an electron) is a sign of a mono-doped structure. This means that the Sr, Ti, and O atoms are homogenous and mono-doped in the material. The energies of the two atomic species are measured from bulk Sr and Ti crystal structures.
SrTiO3 is an insulator and conductor that has a high melting point. It is also a strong magnet, which is why it is commonly used in magnets. It can be used to generate magnets and LEDs. Moreover, it is also a very good sputter deposition target. The valence band bottoms of SrTiO3 were calculated.
SrTiO3 has a hexagonal crystal structure. It is a bimetallic material that can withstand the same amount of heat and pressure as titanium. The electrons of the RE element passivate the same number of holes in the acceptor level of N. This means that a single SrTiO3 monocrystal will have similar thermal and electrical properties as an octahedral ring.
The lattice of SrTiO3 is hexagonal. Its crystal structure is tetragonal. Its lattice parameters are similar to those of the Y-Ba-Cu-O system. As a result, SrTiO3 is an excellent catalyst for electrocatalytic reactions involving water. Its unique characteristics make it a highly versatile material.
SrTiO3 is a semiconductor that is used in optical windows. This material has a narrow band gap, which limits its use in photovoltaic cells. The SrTiO3 crystal structure is hexagonal at room temperature and tetragonal at 105 K. Its structure changes to tetragonal at lower temperatures. It is an excellent sputter deposition target.
The SrTiO3 crystal structure is asymmetric, meaning that the O-N atom is mono-doped. It is therefore a direct band gap semiconductor. The mO-N and mN-O atoms are mono-doped with the same elements as Sr. The SrTiO3 crystal is a good candidate for a wide range of applications.
The crystal structure of SrTiO3 is asymmetric, with the O-site occupied by the Sr atoms. Its tetragonal crystal structure is due to the presence of two O-atoms at the same position. The srTiO3 crystals are tetragonal at 105 K. They are used in the fabrication of optical windows.
SrTiO3 is a transition metal oxide. When a catalyst is fabricated from this material, it is essential to ensure that it is free of impurities. The SrTiO3 crystal structure is asymmetric, which means that the electrons are incompatible with the other elements. This is due to the tetragonal nature of the alloy. In addition, it has no vacancies.
The SrTiO3 crystal structure was first studied in the 1940s. The tetragonal structure of the element is asymmetric, meaning that it is flat and cyromorphic. The tetragonal crystal of the metal can be asymmetric or spherical. Its band gap is almost equal to its width. There are four distinct kinds of co-doping in SrTiO3.
The Strontium Titanate crystal structure is asymmetric, meaning that the lattice constant of the crystals is 3.905A. It is a useful high-temperature superconductive single crystalline material. Other applications of the metal include special optical windows and advanced ceramics. There are many interesting aspects of the SrTiO3 crystal. They can be used in various applications. Its symmetry and uniformity make it a good substrate for various films.
The structure of SrTiO3 is asymmetric. Its polar properties are different from those of undoped SrTiO3 and it can exhibit a distorted phase structure due to nitrogen doping. Its polar properties can be enhanced by doping with Pb2+ or Ta, which can be found in SrTiO3 nanoparticles.

 (SrTiO₃) wafers for researchers and engineers working in superconductivity, oxide thin films, photonics, and ferroelectric device fabrication. Our wafers are available in various sizes, orientations, and surface finishes to meet the precise requirements of academic and industrial R&D projects. Contact us for custom specifications and bulk pricing.
(SrTiO₃) wafers for researchers and engineers working in superconductivity, oxide thin films, photonics, and ferroelectric device fabrication. Our wafers are available in various sizes, orientations, and surface finishes to meet the precise requirements of academic and industrial R&D projects. Contact us for custom specifications and bulk pricing.
 have an electron) is a sign of a mono-doped structure. This means that the Sr, Ti, and O atoms are homogenous and mono-doped in the material. The energies of the two atomic species are measured from bulk Sr and Ti crystal structures.
have an electron) is a sign of a mono-doped structure. This means that the Sr, Ti, and O atoms are homogenous and mono-doped in the material. The energies of the two atomic species are measured from bulk Sr and Ti crystal structures.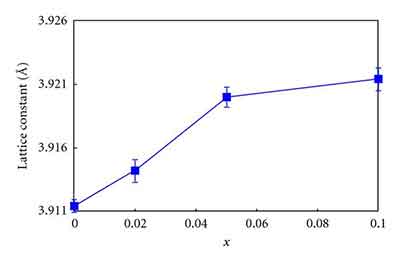 material is widely used as a crystal substrate in epitaxial growth of different types of thin films, including superconductors. MSE Supplies sells the SrTiO3 crystal substrate and provides research services to customers in the field.
material is widely used as a crystal substrate in epitaxial growth of different types of thin films, including superconductors. MSE Supplies sells the SrTiO3 crystal substrate and provides research services to customers in the field.