High Oriented Pyrolytic Graphite (HOPG) for Research
Highly Oriented Pyrolytic Graphite (HOPG)
Recently a scientist asked:
I'm interested in purchasing HPOG to use as a getter in a UHV vacuum system and I have a few questions. 1) Can you source HPOG in different dimensions? I'm looking for pieces that are the size ~5.5x5.5x0.5 mm 2) Is it easy to cut, break, or shave the HPOG down to size?
UniversityWafer, Inc. sold the following:
HPOG to use as a getter in a UHV vacuum system
questions. 1) Can you source HPOG in different dimensions? -- Yes,We can supply different dimensions of HOPG,We can supply the size ~5.5x5.5x1.0 mm ; 10x10x0.5mm; 10x10x1mm;20x20xT(1~2)mm;30x30xT(1.5~2)mm
2) Is it easy to cut, break, or shave the HPOG down to size? --Yes,it not hard to break or shave it down to smaller size
3) HOPG,10x10x0.5mm $ contact us
We also recently sold the following:
1. HOPG 10x10x0.5 mm 10pcs
Get Your HOPG Quote FAST! Or, Buy Online and Start Researching Today!
HOPG is similar with single crystal graphite and graphene.
What is High Oriented Pyrolysis Graphite (HOPG)?
What is High Oriented Pyrolysis Graphite (HOPG)? This article will introduce Graphite, its atomic pattern, and basal plane. The article will conclude with a discussion of HOPG applications and its structure. This is a quick overview of the material's fundamental properties. Graphite is a strong, yet lightweight metal, with high electrical conductivity.
Graphite
For scientific research projects, High Oriented Pyrolytic (HOPG) graphite is a great choice. It has a high diamagnetic strength and is able to float stably in a magnetic field. It can also defy gravity forces up to 16 Tesla. Those properties have led to applications in rockets, solid rocket engines, and frog levitation experiments.
The thermal conductivity of pyrolytic carbon is significantly increased, resulting in higher heat transmission and a longer lifespan. This high thermal conductivity is achieved by segmenting anisotropic pyrolytic graphite into high and low-temperature planes. These planes are then joined with a thermally conductive metal backing by four low-temperature bonding methods: powder metallury compaction, liquid-metal casting, and eutectic brazing.
The high-orientation of HOPG samples, obtained from Advanced Ceramics Corporation, was etched using an oxygen plasma, generated by microwave radiation at a frequency of 2.45 GHz. Two different etching conditions were implemented to create different kinds of pores. After the HOPG was etched, the carbon fibers were separated and processed to obtain the desired result: activated carbon fibers.
Electrochemical etching was also investigated using atomic force microscopy. HOPG is coated with terrace steps, and electron microscopy revealed spots of positive and negative charges around these terrace steps. These spots correspond to the direction of motion of the microscope needle, which determines the magnitude of the charges. Furthermore, the terrace steps function as a nanoscale diode, allowing the surface to conduct electric currents.
What is the Oxide of Graphite HOPG?
The oxidation process of highly oriented pyrolytic graphite (HOPG) leads to significant changes on the surface of the material. These changes can be observed at the cross-sectional face and basal plane. These defects are the result of the deposition of atomic oxygen. These defects are microscopic in nature. The following figure shows the characteristics of HOPG.
HOPG specimens consist of multiple layers of microscopic monocrystal grains. The bulk polycrystal structure is columnar, with columns running vertically in the slab. The lateral surfaces of each grain exhibit grain boundaries. The grains are slightly disoriented with respect to each other. Their c axes are oriented at an angle of about one degree. The specimen's surface has a mosaic-like spread of atoms.
Highly oriented pyrolytic graphite is a high-quality, high-purity form of pyrolytic graphite. Unlike other graphites, HOPG exhibits low mosaic spread angles. Most samples with mosaic spreads of less than one degree exhibit an almost monolayer structure. HOPG can also be exfoliated into thin, 2D layers. These properties make HOPG an ideal substrate for high-end applications.
Various thermophysical properties of pyrolytic graphite were studied by Belikov, R. S., and Senchenko, V. N. The density of pyrolytic graphite was 2.18 grams/cm3. The linear thermal expansion in the basal plane and perpendicular direction were 16.4 A+ 1.6% and sixteen percent, respectively. These results enable the calculation of density at a wide range of high temperatures.
What is HOPG Graphite Basal Plane?
HOPG, or High Oriented Pyrolytic Grade, is an amorphous form of pyrolytic graphite that is produced by applying an additional tensile stress in the basal plane direction. This produces a more uniform, highly ordered structure and allows for the production of graphite with an interplanar spacing that is close to that of natural graphite. The high-purity structure of HOPG has made it useful for x-ray diagnostics and spectroscopy.
HOPG has an unusual basal plane that can be used for energy storage. The surface of HOPG has terrace steps that act as nanoscale diodes. Electron transfer in the basal plane of HOPG is slow, resulting in a large DEp value. The HOPG basal plane also exhibits a large range of charge distributions due to electron transfer.
The thermophysical properties of pyrolytic graphite have been studied by Senchenko, V. N. and Belikov, R. S. The density of pyrolytic graphite is 2.18 g/cm3, and its linear thermal expansion in both directions is 16.4 A+ 1.6%. These results enable calculations of density for high temperature applications.
High Oriented Pyrolytic Grade (HOPG) consists of a 0.5-A thick layer of porphyrins covering the graphite surface. The topography of this layer is acquired before EC in H2SO4. The white dashed line represents the cross-section profile. The blue areas represent the porphyrin wetting layer, while the yellow areas are the HOPG regions. Interestingly, no blisters are seen in region A.
What is Graphite Atomic Pattern
For research purposes, High Oriented Pyrolytic-Graphite (HOPG) is a superior material. Its high purity and low impurity level make it a superior choice for advanced scientific research. The atomic pattern of HOPG allows for a wide variety of processes, from magnification calibration to x-ray optics. The following are some of the benefits of HOPG.
The asymmetric positive contrast of the HOPG atomic pattern is evident from the observations that bright spots originate from three of six C atoms in the hexagonal unit cell. This is due to the fact that every apparent atom has six nearest neighbors, which results in threefold symmetry. Furthermore, the asymmetric atom environment is reflected in the crystalline structure.
The surface of highly oriented pyrolytic graphite contains terrace steps, which can be used to study its atomic structure. The atomic pattern is composed of spots of positive and negative charges in close proximity to each terrace step. The positions of these spots depend on the speed and direction of the microscope needle. This makes the terrace steps a nanoscale diode, allowing surface electric currents to flow through the graphite.
HOPG is the highest three-dimensionally ordered material and exhibits similar physical properties to the natural graphite mineral. It belongs to the lamellar group of materials. It has a lamellar crystal structure, in which carbon atoms are stacked in parallel layers. Similar to graphene, HOPG has a strong interplanar interaction, which is responsible for graphite's cleaving behavior.
What is HOPG Graphite Intercalation?
A novel mechanism of intercalation was discovered in HOPG. High voltages for less than Dt = 4 s induce a carbon dissolution reaction. During this process, graphite terraces are peeled off layer by layer. The intercalation of ammonium ions into graphite improves its puckered effect, out-of-plane deformation, and friction. The intercalant molecules are small in size, resulting in higher interlayer friction and roughness.
Binary-element intercalation of graphite leads to a superconducting compound. This compound has a very low T c onset, whereas the CaC6 superconductor has the highest T c onset. In a ternary-element graphite, however, the elements Cs, Yb, and Sr form a superconductor.
HOPG and GICs are useful in composite lubrication applications. The latter material provides increased ion density and puckered effect. Moreover, GICs are used as graphite additives and composite lubricants. AFM spectra analysis has shown that HOPG and graphite intercalation can coexist in nanostructured devices.
Electrochemical intercalation of graphite is an extremely simple, effective, and inexpensive method. This method is especially advantageous because it uses graphite cathodes that are inert in aqueous solution. The cathodic reduction process can occur even in the presence of water electrolysis potentials. The aprotic medium, however, is stable during cathodic reduction, and is hardly affected by large negative potentials. It is also possible to use non-aqueous electrolytes, which are highly soluble in organic solvents.
What is HOPG Graphite Thermal Conductivity?
The surface of a high-orientation pyrolytic graphite (HOPG) sheet exhibits high thermal conductivity. This material is stable because p-p interactions stabilize the molecules adsorbing on its surface. In addition to aromatic molecules, long-chain alkanes can also be adsorbing parallel to HOPG surfaces. The space between the methylene groups of the alkane molecules is almost equal to the hexagon spacing of the graphite surface. The van der Waals interactions between molecules and surfaces on graphite facilitate the formation of a monolayer of alkanes.
HPMS pyrolytic graphite sheets are extremely thin, allowing easy attachment to heat sinks and component surfaces. These properties enable them to be used in many applications, from cooling to scientific measurement equipment. In addition, because HPMS pyrolytic graphite sheets are thin, they are flexible and can be used in wide temperature ranges. However, they are not as versatile as copper or other traditional thermal conductivity materials.
After graphitization at 3000degC, amorphous PI film is formed, with a few microcrystals of graphite on the surface. The ratio of these two peaks reduces over time, and the D peak completely disappears once graphitization has reached 2400degC. In contrast, amorphous carbon and structural defects are low in a graphite film with a large crystalline structure. The microcrystalline size depends on the nature of the PI film and the thickness of the graphite film. The thicker the film, the lower the preferred orientation.
Video: Highly Oriented Pyrolytic Graphite
High Oriented Pyrolytic Graphite for Research
High Oriented Pyrolytic (HOPG) graphite is a layered polycrystal. The bulk polycrystal is composed of columns that run vertically in a flat slab. The lateral surfaces of grains have disoriented orientation. The c-axes of the crystallites are disoriented by about 1 degree. The surface of HOPG consists of multiple randomly placed atomic steps, which can have multiple atomic layers.
HOPG is characterized by a high degree of three-dimensional order, making it similar to 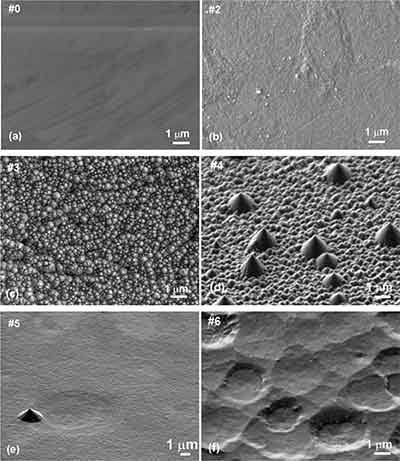 the physical properties of natural graphite. It is classified as a lamellar material because it has layers of parallel carbon atoms that are aligned to form a sheet. The grain boundaries are stacked in a way similar to a graphene sheet. The strong interplanar interactions between the atoms of HOPG are what allow it to exhibit its cleaving behavior.
the physical properties of natural graphite. It is classified as a lamellar material because it has layers of parallel carbon atoms that are aligned to form a sheet. The grain boundaries are stacked in a way similar to a graphene sheet. The strong interplanar interactions between the atoms of HOPG are what allow it to exhibit its cleaving behavior.
High Oriented Pyrolytic graphite is an excellent choice for research projects. This material's impurity level is less than 10 parts per million. Its flat surface is a good candidate for many applications, including abrasive and electronic surfaces. It also allows researchers to do a wide variety of experiments with a variety of materials. The HOPG is a superior choice for advanced scientific work, and it can be used in a wide range of processes.
HOPG is an excellent choice for research. Its purity and order make it ideal for a range of high-end applications. Its mosaic spread angle is less than 1 degree. Its layers are linked together through van der Waals interactions and can be exfoliated into thin, 2D layers. This graphite is commonly used in x-ray optics as a monochromator, and in scanning probe microscopy as a magnification calibration substrate.
HOPG is the most pure and ordered form of synthetic graphite. It is a perfect choice for use in x-ray spectroscopy, and other applications. This material is a highly efficient way to improve the quality of x-ray images and to reduce impurities. The high-oriented pyrolytic graphite is highly resistant to heat, light, and mechanical shocks.
HOPG is an extremely pure and highly ordered form of graphite. Its impurities are less than ten ppm. Its structural features are similar to natural graphite, and HOPG is useful for research. It is also used in the fabrication of nanometer-scale precision tools. The HOPG is a great candidate for high-resolution imaging.
HOPG is a highly ordered form of pyrolytic graphite. It is one of the few materials with a low impurity content. It is a renewable, easy-to-use material. For researchers, HOPG provides an inexpensive, flat and clean substrate that is ideal for SPM measurements. Its high-resolution graphene is an essential ingredient for a wide variety of research.
HOPG is an excellent choice for x-ray applications. Its high-purity and low-ash content make it an excellent material for x-ray spectroscopy. In addition, HOPG is also a good choice for a number of other uses. It can be used in a variety of different applications. This highly ordered graphite is available for research purposes.
HOPG is an extremely pure form of synthetic graphite. Its hexagonal structures are highly ordered and uniform, and the mosaic spread angles are very low. Its crystal structure is also similar to graphene, which is a lamellar material. The hexagonal-shaped crystals are stacked in parallel planes and can be exfoliated into thin 2D layers.
The surface of HOPG is suitable for DNA functionalization. In addition to its high atomic-force microscopy, bare HOPG surfaces induce the formation of parallel ssDNA. The resulting nanoparticles display the structure of DNA molecules. This type of material is an excellent substrate for a wide range of research activities. Its nanoparticle size is suitable for the production of semiconductor devices.
The surface of HOPG is patterned by a method called electrografting. A supporting electrolyte containing aryl diazonium salts was applied to the graphitic surfaces. AFM and scanning tunneling microscopy images revealed circular spots on the graphitic surface. These spots were believed to be nanocorrals, originating from near-surface bubbles.
What is Highly Oriented Pyrolytic Graphite HOPG?
Electrochemical insights have revealed a new way of defining graphene - like graphite electrodes that can be printed on a screen with high precision and high energy efficiency. [Sources: 4]
A range of technologies have been developed to take advantage of the perfect graphite sample with a unique structure. These systems are based on highly oriented pyrolytic graphite as a substrate for metal deposition (np). However, most measurements are made with highly oriented pyrolysis graphs, a very pure and ordered form of graphics with a high surface area and high electrical conductivity and high energy efficiency. [Sources: 0, 1, 5]
In this plot it becomes clear that most experimental data is consistent with the theory of Sharifker et al. G - Point vibrations, which optically enable a high surface area, high electrical conductivity and high energy efficiency, are shown in Fig. HOPG is therefore a high-order form of graphite with a very pure, highly conductive and highly energy-efficient structure. The contamination values are in the order of 10 ppm (ash - well, yes), which is about as high as natural graphites and minerals. [Sources: 0, 6, 7]
The characteristic features of SPM images can be easily interpreted in the form of "A" and "B" stacked with graphite. [Sources: 0, 8]
Note that there is a big difference between "A" and "B" in the size of the graphite, and the smaller "C" is smaller. [Sources: 6]
Compared to the underlying substrate Au-111, the molecular sequences are parallel to 110 in both directions. Compared to the substrate below: molecular rows in the direction of 110, but not in every direction. [Sources: 2]
The HOPG - terminated graphene layer is the aim of scanning probe microscopy, which is used as a substrate calibration standard for atomic resolution. It is interesting that due to the mosaic spread of the HopG crystals, it is possible to record the spectrum in a much higher resolution than with natural graphite. The improved alignment of the molecular sequences in the graphene layers results in improved arrangements, resulting in a high resolution spectrum and a wide wavelength range, similar to that of graphites, but with the characteristic mosaic, accompanied by mosaic focusing and high integral reflectivity as used in H HopG. [Sources: 0, 8]
HOPG is chemically similar to graphene and can be used in a wide range of applications such as nanoscale imaging and high-resolution spectroscopy. Support for individual nanoparticles and nanoparticle ensembles for electrocatalytic investigations. HOP Graphene - Insulated Ultramicrometer with Glass - Insulated carbon fiber electrodes for high resolution scanning probe microscopy and spectra. [Sources: 4, 8]
N - modified HOPG for electrochemical scanning electron microscopy and high resolution spectroscopy of nanoscale structures. A carbon-gold film produced with electron beam evaporation for high-resolution scanning electrochemicals and spectra of a graphene nanoparticle. [Sources: 4]
A metal layer deposited by thermal evaporation, as shown in Fig. 2, and a resistant layer of graphene with high-resolution spectroscopy spectra of a graphene nanoparticle. N - modified HOPG for electrochemical scanning electron microscopy and high-resolution scanning electron microscopy using graphene nanoscale structures for high-resolution scanning electrochemicals and spectrometry. A carbon-gold film with graphene nanonanometer structures is produced by evaporation of the electron beam and thermal deposition of the graphene film on a copper oxide substrate. [Sources: 2, 3]
Electron microscopic inspection of the manufactured columns shows negligible etching of the graphite columns on the metal mask (see Fig. Finally, we were able to show that SEI is better bound to the rough surface of battery-grade graphites than the flat surfaces of HOPG. The measured data correspond to the classical diffuse transport model, which characterizes sample19. [Sources: 3, 5]
The D and G energy calculated for the formation of stable nuclei is 8 - 21x10 -21 J / nucleus. Due to the presence of high-energy electrons in the graphite surface of HOPG, it is not possible to detect the formation of cathodic peaks. [Sources: 7]
To solve this problem, we embedded the particles in an epoxy resin and made cross-sections so that they are firmly bonded to the substrate, "says Luchkin. Cylindrical graphite columns were produced from freshly cleaved HOPG substrates by anisotropic oxygen plasma etching. [Sources: 3, 5]
The STM tip of the gold sample was prepared by electrochemical etching with 12 - 15 V. The substrates were used for the first time in anisotropic oxygen plasma etching at a temperature of 1,000 degrees Celsius. After 12 to 15 hours, the caustic process was stopped and the substrate was reused. [Sources: 2, 3]
The microstructure of the electrobearing was investigated using 111 gold single crystal beads produced by melting gold wire 99 - 999. To investigate the effects of substrate and adlayer formation, molecular adlayers were produced in highly oriented, high-temperature, low-pressure, liquid-oxygen plasma etches. Atomic Force Microscopy (AFM) was used and images were obtained with the Jeol JSPM 4210 microscope. [Sources: 2, 7]
Under ideal conditions, the SPM images of the HOPG surface showed the presence of a hexagonal ring of six C atoms in the center of each spot on the surface. The fact that the six C atoms are made up of hexagonal ring-shaped points around them gives a bright signal that leads to the formation of molecular layers in a highly oriented, high-temperature, low-pressure, liquid-oxygen plasma-corrosive plasma. [Sources: 0]
Sources:
[0]: http://nanoprobes.aist-nt.com/apps/HOPG%20info.htm
[1]: https://escholarship.org/uc/item/23d1g39v
[2]: https://www.pnas.org/content/102/4/971
[3]: https://www.nature.com/articles/ncomms6837
[4]: https://pubs.acs.org/doi/10.1021/ja308615h
[5]: https://www.innovations-report.com/power-and-electrical-engineering/skoltech-scientists-get-a-sneak-peek-of-a-key-process-in-battery-life/
[6]: https://www.sciencedirect.com/topics/engineering/highly-oriented-pyrolytic-graphite
[7]: https://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-40422010000500019&lng=en&nrm=iso&tlng=en
[8]: https://www.alfa-chemistry.com/products/highly-oriented-pyrolytic-graphite-44.htm
