Solar Silicon for University Researchers
Get Your Quote FAST!
High Efficiency Concepts of c-Si Wafer Based Solar Cells
Advances in Solar Silicon Efficiency
The current record for solar cells using silicon is 26.7+0.5%, which is already close to the limiting value of 29.1%. But further improvements are needed to improve the efficiency of silicon-based solar cells. The most promising tandem structures are perovskite/silicon multijunctions and III-V/silicon multijunctions. But further advances are needed before these can be implemented in commercial applications. Nevertheless, these advancements are still a long way off.
For example, one program for simulating the classic n+pp+ structure uses the silicon solar cell parameters as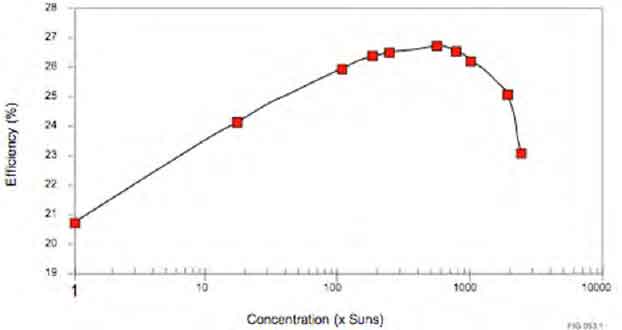 p-type wafer with a depth of 5 mm and a density of 1 x 1016 cm-3 of phosphorus. It simulates a n+ front phosphorus diffusion and the rear surface recombination velocity of 104 cm s-1. But it is not clear whether or not these parameters can help a PV panel's efficiency.
p-type wafer with a depth of 5 mm and a density of 1 x 1016 cm-3 of phosphorus. It simulates a n+ front phosphorus diffusion and the rear surface recombination velocity of 104 cm s-1. But it is not clear whether or not these parameters can help a PV panel's efficiency.
The maximum conversion efficiency of solar cells based on c-Si is about 29%, although it can increase to 30% depending on the type of material used. Optimal cell configurations depend on the back surface recombination velocity SB and the thickness of the cells. The calculation of these parameters is based on the FEM. This method is known to be more accurate than simulations. But it can't fully explain all the details of the system.
In conventional light trapping structures, the Lambertian limit cannot be reached. In addition, optimum cell thicknesses are higher than 110 mm and a thin silicon layer is best for efficient carrier collection. Moreover, the non-zero bulk doping of the silicon layers reduces the theoretical power conversion limit by at least a percentage point. The recombination limits for the maximum conversion efficiency are not achievable when the ray-optic method is applied.
Despite the fact that solar cells are widely used in many commercial applications, their efficiency is limited by several factors. The optimal thickness of the silicon layer is dependent on the trade-off between high optical absorption and low optical absorption. The PERL limit is the most significant limit that limits the conversion efficiency of a solar cell. The best cell is one that is patterned in a pattern that is highly optimized for light-trapping and carrier collection.
A single-cell model is usually a good representation of a single solar cell's characteristics. A model can be derived from the resulting results of an n-type solar cell. Another type of cell can be modeled using the N-type monocrystalline silicon. The P-type cell is more efficient than a polycrystalline silicon cell. It has higher efficiency than its n-type counterpart. A thin-film c-Si solar cell can have a varying density of photogenerated carriers.
An ideal cell has an open-circuit voltage that is lower than the open-circuit voltage. In addition to this, it has a higher fill-factor and is more efficient than other solar cells. However, it has an inferior efficiency compared to polycrystalline silicon. A silicon solar cell is not an ideal device, because its efficiencies are limited by the recombination of light. Nonetheless, a single-junction solar cell can be a good candidate for solar energy conversion.
The optimal thickness for a solar cell depends on the trade-off between high optical absorption and low light-trapping. A thin silicon layer is ideal for efficient carrier collection. For best results, the cell should be more than 110 mm thick. A thin layer is also necessary to improve the light-trapping and trapping of the light. Besides, an ideal silicon cell should have the best optical absorption and maximum light-trapping properties.
The efficiency of a silicon cell depends on the materials it uses. The base silicon material is typically N-type or P-type. The fill-factor is the maximum conversion efficiency of the PV cell when operating at the optimum voltage. Other factors that influence the panel efficiency are the type of silicon, the busbars, and the passivation type. The most efficient cells are high-cost mono PERC cells with an N-type silicone base and no busbar shading.
The open-circuit voltage of a silicon solar cell is approximately inversely proportional to its thickness. If the illumination is cut by 80%, the open-circuit voltage drops by ten percent. The SRV of a low-quality cell is less than one half of that. In order to produce usable power, a solar cell's efficiency must be more than twice its thickness. If the SRV is too high, the solar cell is useless.
Solar Silicon Cells up to 19.5% efficiency
Since the 1970s the National Renewable Energy Lab (NREL) has cataloged over 20 differerent type of solar cell technology. Below are some solar cell technologies:
- traditional crystalline silicon cells
- thin-film cells
- single-junction cells
- multi-junction cells
- quantum dot cells
- solar concentrators
These above tech have various efficiencies.Our monocrystalline solar cells have efficiencies of up to 19.5%.
What does solar efficiency rating measures the percentage of sunlight photons hitting the solar cell for a given space. Higher efficiency means that less surface area is required to to generate electricity. So a small rooftop will want to use higher efficiency panels to compensate for smaller surface area.
Below are just some of the Solar cells that we have in stock.
At present, we had poly and mono solar cells in stock as follow.Hope it is useful to you.
6 inch Poly cell
- - Poly solar cells,156*156mm,6inch,3BB/4BB,17.6-17.8% efficiency
- - Poly solar cells,156*156mm,6inch,3BB/4BB,above 18% efficiency
6 inch Mono cell
- - Mono solar cells,156*156mm,6inch,3BB,4.3Watt/PCS
- - Mono solar cells,156*156mm,6inch,3BB,18.8-19.5% efficiency
5 inch Mono cell
- - 125mm Mono Solar cells ,P type, 2BB, 2.8W/PCS
- - 125mm Mono Solar cells ,Sunpower, 2BB, 3.2-3.5W/PCS
What is the Source of Creation in a Solar Cell?
The creation of energy happens through the CNO cycle. Electricity is created when the negative and positive sides of a solar cell are connected together through a connector. This connection makes electrons loose and they move toward the front surface of the solar cell. This creates a vacancy in the cell and creates a current. The flow of electricity is known as photovoltaic conversion.
electrons are loosened
In a solar cell, sunlight hits the front surface, knocking electrons loose. The newly-liberated electrons move away from the back and toward the front. The back surface is treated with a thin film of conductive material to keep the hole formed there. When sunlight strikes the cell, the electric field pushes the freed electrons towards the top layer, where they are slingshotted to a strip of metal conductor.
The process of releasing electrons from a material called a solar cell involves knocking these extra electrons out of the cell's material. In solar cells, this happens when sunlight strikes a layer made of silicon. This layer contains P-type silicon, which contains free openings. This layer holds a negative charge. The other layer is positive, or p-type. Both types of solar cells have the same properties, but in different ways.
When sunlight strikes in the solar cell, the silicon layers are bombarded with a strong electrical field. As the electrons are knocked loose, they move toward the silicon's conductive surface. This action generates an electrical current, which is captured by the wiring of the solar panel. This electricity is then converted into usable electrical energy. With this technology, people can generate electricity for free.
When sunlight strikes in the solar cell, electrons are knocked free of their bonds. As a result, the energy of the light strikes the semiconductor and knocks electrons loose from their valance levels. When sunlight strikes the cell, billions of photons strike the device, knocking the electrons loose and causing them to flow. Afterwards, the energy is transferred into useful electricity.
They move toward a treated front surface
Electrons on the n-type side of the solar cell absorb energy from the sunlight and break free of their atoms. These excited electrons travel along the wire to the p-type side and provide electrical power to the electronics during their journey. The high-energy light hits this semiconductor, which converts the energy into electricity. In the process, it creates a small hole.
The sun's heat and pressure cause the photons to reach the solar cells. These photons carry solar energy from the sun's core. The sun's constant nuclear fusion reactions create massive amounts of energy. This fusion creates helium and hydrogen atoms. The sun also produces more than 500 million tons of hydrogen atoms every second. Despite the fact that only 2% of the energy is created by nuclear fusion, the energy created by the sun is enough to sustain all plant and animal life on the planet.
A photovoltaic solar cell contains two layers of silicon, one negatively charged and the other positively charged. Light from the sun energizes the silicon wafers and causes electrons to be freed from their atoms. This freed electron motion causes an electrical current. The electrical current is then created when the negative side and positive side are connected by the connector. This creates a current of energy that flows between the cells.
The process of electricity production in a solar cell involves electrons being transported from the sun to the back surface. The material of the solar cell is porous and absorbs enough photon energy to dislodge electrons and migrate to the front surface. The front surface of the solar cell is specially designed to be more receptive to free electrons. When two surfaces of the solar cell are connected to each other through an external load, electricity flows.
Electricity occurs when a connector joins the negative and positive sides together
An individual solar cell contains two different types of semiconductors: n-type and p-type. When p-type silicon is added to an n-type cell, it forms an electron vacancy. When sufficient sunlight hits the n-type cell, the atoms collide and produce an electrical current. This process is called photovoltaic. Electricity is generated between the two surfaces of the solar cell.
A photovoltaic cell converts the energy in sunlight into electricity by causing an electrical current. The electricity generated by a PV cell has zero carbon dioxide emissions. The photon energy absorbed by nanomaterials in the solar cell is transferred to electrons inside the atoms. The PV cell's operation is illustrated in Fig. 41.1.1. Electrons and holes wander across the junction to form the electric flow.
When a solar cell is connected to an electrical circuit, the n-type semiconductor will become the adjunct of the p-type. In this way, the n-type semiconductor becomes a p-type semiconductor and the positive side will be a p-type semiconductor. The other electrical contact in the diode is formed by a metallic layer attached to the back surface of the solar cell.
The basic structure of a p-n junction is illustrated in Fig. 23.6. The p-n junction is where the two sides of a solar cell will connect. Fig. 23.6 shows the basic structure of a PV cell and how electricity flows through the cell. In addition, a solar panel can be used for home or business purposes.
The CNO cycle drives the creation of energy
The process of converting hydrogen into helium is driven by a reaction called the proton-proton exchange. All constituents of the gas are consumed in the reaction, including carbon, oxygen, and nitrogen. Carbon is consumed and regenerated in the process, resulting in two electron neutrinos and an alpha particle. A photon emitted during this reaction creates energy for the solar cell.
The sun's energy is derived from the proton-proton chain reaction, a process that creates an enormous amount of energy and heat. Stars about the size of our sun undergo this same process to create heat and light. It is this continuous energy transfer that sustains life on earth. The temperatures involved in the reaction are around 4 million degrees Kelvin. This energy is then stored in the form of electricity.
The energy produced by the CNO cycle can be harnessed in many ways, including the production of electricity, heat, and light. A solar cell can convert solar energy by using photovoltaic panels, solar radiation concentrating mirrors, and thermal storage. The energy generated is usable and can even be stored in batteries and thermal storage. In the long run, this energy can make a huge difference in the environment.
Video: How do Solar Cells Work?
