Can you fabricate a custom stack involving ITO, amorphous hydrogenated silicon and SiO2? I do not see a-Si-h listed on your website. We just need some simple, maybe ideally straight, waveguides for some tests. The details shouldn't matter too much. For the amorphous silicon custom device, yes we would have precise requirements on thicknesses."
Amorphous Silicon Used in Research
A scientist asked the following:
Please reference #250469 for specs/pricing.
Get Your Quote FAST! Or, Buy Online and Start Researching Today!
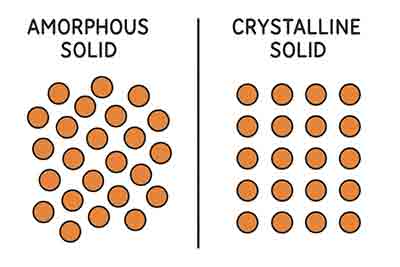
5 Differences Between Amorphous & Crystalline Solids
Below are 5 differences to consider when choosing to research amorphous & crystalline solids.
Crystalline Solids
- Crystalline Solids have an orderly arrangement of their constituent particles.
- Crystals have a SPECIFIC GEOMETRIC SHAPE WITH DEFINITE EDGES.
- Crystalline solids cleave along particular points & directions.
- Crystalline solids have a sharp melting point.
- They are also known as true solids.
Amorphous Solids
- Amorphous solids have no such arrangement. Their particles are randomly organized.
- They do not have geometry in their shape.
- Amorphous solids cleave into uneven parts with ragged edges.
- They have a range of temperature over which they will melt.
- They are also known as supercooled liquids.
Characteristics
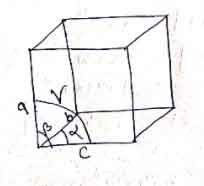 The following characteristics define a unit cell:
The following characteristics define a unit cell:
- A unit cell has three edges a, b & c and three angles α, β & γ between the respective edges.
- The edges a, b & c may not be mutually perpendicular.
- The angle between edge b & c is α, between a & c is β, and between a & b is γ.
Determining The Difference Between Crystalline & Amorphous Solids
The fundamental difference between crystalline and amorphous compounds is the arrangement of their constituent atoms. A crystalline solid has a long range of ordered molecules and a sharp melting point. In contrast, an amorphous compound has a short range of ordered molecules and an irregular arrangement of its atoms. This makes amorphous compounds highly rigid with irregular surfaces.
Amorphous solids are non-crystalline substances that do not possess a characteristic geometrical arrangement. They also do not have a fixed melting point. Despite their name, amorphous materials do display an orderly arrangement of atoms that may extend up to a few Angstrom units. Such structures are called crystallites.
Amorphous & Crystalline Solids Lecture
What Does Amorphous Mean?
What does Amorphous mean? This term describes a solid that lacks definite form or pattern. It is characterized by the lack of pattern, structure, or organization. As a result, it is difficult to define.
In chemistry, amorphous materials are those that lack a crystal structure, meaning that their structure is irregular. The term "amorphous" comes from Greek. Because amorphous materials do not possess a geometric shape, they do not have sharp melting points. These materials are widely used in many fields, including plastic, rubber, polymers, and other household materials.
Amorphous Materials
What are Amorphous materials? They are solids that lack long-range order. They were once used to describe glass. However, these solids have no crystal structure and are typically not crystalline. We'll discuss amorphous metals and glass.
Amorphous solids are common types of solids. They don't have any crystalline structure and have no ordered or periodic structure. Many of the solids we use in our daily lives are amorphous, such as plastics.
What is The Definition of Amorphous?
The term "amorphous" refers to something that lacks a defined shape or structure. It is used to describe a substance or material that does not have a regular, repeating pattern of atoms or molecules, and therefore lacks a distinct crystalline structure. Amorphous materials can be solids, liquids, or gases and can range from simple molecules like water to complex polymers like plastics. Unlike crystalline materials, which have a well-defined arrangement of atoms or molecules, amorphous materials have a more disordered or random arrangement. Examples of amorphous materials include glass, rubber, and some types of metals.
Examples of Amorphous Solids
Amorphous solids are characterized by a lack of long-range order in their atomic or molecular structures. Here are some examples:
Glass: One of the most common examples, including window glass, glass containers, and fiberglass.
Plastics: Various types of polymers used in everyday items.
Gels: Substances that exhibit properties between those of liquids and solids, like gelatin.
Rubber: Used in tires, footwear, and various flexible products.
Amorphous Metals (Metallic Glasses): Alloys cooled rapidly to prevent crystal formation, used in electrical applications and high-strength materials.
Thin Film Coatings: Used in optics and electronics, such as anti-reflective coatings.
Pitch: A viscous, tar-like substance.
Silicon Dioxide (Silica) in Certain Forms: Such as fused silica used in optics.
Some Types of Wax: Like paraffin wax.
Foams: Polyurethane foam used in mattresses and insulation.
Thermal Oxide Grown on Amorphous Silicon Wafers
A postdoc requested a quote on the following:
"I am looking for amorphous silicon grown on top of SiO2 on silicon substrate. Whatever thickness and diameter you can provide with shortest lead time please provide a quote. If we can do 200mm we would need 10 wafers. In terms of specs, we are not too concerned. Let’s say 3kA SiO2 on the (100) Si substrate. And for the amorphous silicon please let me know what you can do for thicknesses. Is 3kA possible? Would price depend on amorphous si thickness?"
Two more questions:
- Would 1kA SiO2 vs 3kA SiO2 impact the price? We are ok with 1kA SiO2.
- If we reduce lot size to 5 wafers will it impact price per wafer?
UniversityWafer, Inc. Replied: Reference # 271938 for specs and pricing.
What is the difference between crystalline and amorphous materials?
Crystalline and amorphous materials are two different types of solids that have distinct structural and behavioral characteristics due to the arrangement of their constituent atoms, molecules, or ions.
Response to Heat
- Crystalline materials: They generally have a well-defined, sharp melting point. As the temperature increases, thermal energy breaks the lattice bonds.
- Amorphous materials: They do not have a sharp melting point. Instead, they undergo a glass transition, softening over a range of temperatures from rigid to viscous.
Response to Pressure
- Crystalline materials: Can exhibit anisotropic mechanical properties (varying by direction). They can undergo phase transitions or deformation depending on the crystal structure.
- Amorphous materials: Generally exhibit isotropic properties (same in all directions). They tend to deform more uniformly under pressure.
Comparison Table
| Property | Amorphous Solid | Crystalline Solid |
|---|---|---|
| Definition | Lacks a crystalline structure; random arrangement. | Ordered structure and symmetry. |
| Repeating Unit | No repeating units. | Distinct repeating patterns (unit cells). |
| Melting Point | No sharp melting point (softens over range). | Sharp, definite melting point. |
| Nature | Isotropic (properties same in all directions). | Anisotropic (properties vary by direction). |
Amorphous And Crystalline Solids Summary
Amorphous solids, often referred to as pseudo-solids or supercooled liquids, lack long-range order. This means their properties are generally isotropic. In contrast, crystalline solids have a definite geometric shape and long-range order, leading to anisotropic behavior.
The main difference lies in the cleavage property: crystalline solids cleave along specific planes creating smooth surfaces, whereas amorphous solids break irregularly with jagged edges.
##### Sources #####
[0]: https://www.britannica.com/science/amorphous-solid
[1]: https://pediaa.com/difference-between-amorphous-and-crystalline-solids/
[2]: https://pubs.acs.org/doi/10.1021/acs.jpclett.8b01067
[3]: https://www.vedantu.com/chemistry/difference-between-crystalline-and-amorphous-solid
[4]: https://chembam.com/definitions/crystalline-vs-amorphous/
[7]: https://en.wikipedia.org/wiki/Amorphous_solid
