I would like some of your "low price" diced undoped silicon wafers - 10mm x 10mm x 0.525 micron SSP, <100> Undoped, >10,000 ohm-cm" for Positron annihilation spectroscopy. Please provide a quote for 20 of those!
What Wafer Specs Should I use for Spectroscopy?
A graduate student from a materials science lab requested a quote for the following:
Please reference #270386 for pricing.
Get Your Quote FAST! Or, Buy Online and Start Researching Today!
Key Spectroscopy Keywords
Below Terms are Associated with Spectroscopy:
- spectroscopy employ
- absorption spectroscopy
- spectroscopy techniques
- spectroscopy uses
- ir spectroscopy
- spectroscopy experiments
- spectroscopic techniques
- field spectroscopy
- spectroscopy measures
- radiation absorption
- absorption spectrum
- spectroscopic devices
- wavelength range
- spectral resolutions
- component wavelengths
What is Spectroscopy?
Spectroscopy is the study of how matter interacts with electromagnetic radiation. It encompasses a variety of techniques used to identify chemical composition and analyze material properties, including Raman, FTIR, UV-Vis, and emission spectroscopy. These techniques often require specialized substrates or wafer materials to ensure high fidelity in measurement and analysis.
Common Wafer Materials for Spectroscopy
- Silicon: Ideal for FTIR and photoluminescence due to high infrared transparency.
- Quartz: Widely used for UV/Visible spectroscopy due to excellent optical transmission.
- Sapphire: High durability and UV transparency, suited for rugged environments.
- Fused Silica: Preferred for laser-based spectroscopy requiring minimal absorption.
- Gold-Coated Silicon: Enhances Raman signals for Surface Enhanced Raman Spectroscopy (SERS).
Applications and Compatibility
UniversityWafer supplies wafers compatible with key spectroscopy modalities:
- Raman Spectroscopy – gold/silver coated substrates
- FTIR Spectroscopy – undoped float-zone silicon or quartz
- Emission/Photoluminescence – silicon, gallium arsenide
- UV/Vis – quartz and fused silica
Spectroscopy Comparison Table
| Feature | Photodiode | Phototransistor | Solar Cell |
|---|---|---|---|
| Mode of Operation | Photovoltaic or Photoconductive | Photoconductive | Photovoltaic |
| Output | Current | Amplified Current | Voltage/Current |
| Sensitivity | Moderate | High | Varies by illumination |
| Typical Use | Light detection | Amplified light sensing | Power generation |
Raman Micro-Spectroscopy
A PhD from a large university requsted that we quote the following:
This may be useful for us to characterize our custom-built Raman micro-spectroscopy setup in my lab. Are the thin wafers mounted on a substrate? Can you clarify what are some of the column headings e.g. TTV, Fz/CZ, Ring/ Freestanding and MOQ.
Please reference #268924 for pricing.
Another scientist requested the following quote:
I would like a thin silicon wafer that has a thickness of around 1 um to deposit on another wafer to make a 1 um deep well for Raman spectroscopy analysis. Rectangular shape is preferred; the smaller the wafer's size, the better since I have to put it on another wafer. I'm curious about the smallest thickness that is available.
Please reference #268359 for pricing.
Frequently Asked Questions
What types of wafers are used in spectroscopy?
Silicon, quartz, sapphire, fused silica, and gold-coated silicon are commonly used based on the specific wavelength range and optical properties needed.
Which spectroscopy techniques require specialized wafer substrates?
Raman spectroscopy benefits from metal-coated wafers for signal enhancement. FTIR requires low-absorption materials like undoped silicon or quartz. UV applications benefit from transparent materials such as fused silica.
The image shows a beam of light being dispersed into its constituent spectrum of colors by a prism, surrounded by various types of spectroscopy equipment in a laboratory setting. This visual aims to highlight the science of studying the interaction between matter and electromagnetic radiation.
Elastic and Reflection Spectroscopy
Spectroscopy techniques are based on the properties of elastic scattering, and are used to examine biological tissues and cells. The properties of light elastically scattered in tissue are dependent on architectural features of the cells and tissues. These features include crystallinity, extended fine structure, and amorphization. Some pathologies exhibit architectural changes at the cellular level. The properties of elastically scattered light can be used to detect abnormalities in these tissues.
Elastic and reflection spectroscopy have been developed as a means to study cell and tissue architecture. These techniques can be used to detect abnormalities in tissues at an early stage. They can be used to identify abnormal protein packages, enlargement of cell nuclei, and morphological changes in epithelial surface texture. They can also be used to detect high grade dysplasia and cancer in Barrett's esophagus.
The elastic and inelastic spectra of light scattered from tissues can be used to identify early abnormal tissue. These spectra can be obtained from different types of samples, such as surgically removed tissues, blood samples, or tissue samples that have been excised. However, spectral differences between normal and abnormal tissue can be subtle.
The elastic peak of the electron energy loss spectrometer spectroscopy is associated with the elastic reflection coefficient re of the sample. This coefficient is determined by the E energy. Unlike inelastic scattering, the elastic reflection coefficient is independent of the incidence angle. It is also determined by physical processes such as refraction, recoil, and extended fine structure.
The low energy electron energy loss spectrum contains an elastic peak. This peak can be used to determine dielectric properties, band structure, and physical parameters of the sample. It can also be used to determine the life span of hot electrons. It is also used to measure the surface excitation parameter of a sample.
The low coherence enhanced backscattering method exploits the coherent effect to isolate light scattering with a specific penetration depth. It has been used to assess the risk of colon cancer. In addition, it has been applied to detect precancerous lesions in the respiratory tract.
An experimental study on excised tissue was done to determine the source of variability. The study investigated possible sources of variability, including angle, pressure, and amorphization. However, further studies are needed to determine reproducibility.
Near-infrared Spectroscopy
Using a diffraction grating, near-infrared spectroscopy separates light into absorption bands. These bands are based on combinations of vibrational frequencies of molecules. The light is then absorbed by the sample. This absorption depends on the type and composition of the sample. Light in the NIR region has a much wider spectrum than the IR range. This makes it useful for studying materials that have high moisture content or density.
In the NIR range, molecules are formed by the stretching-bending vibrations of atomic groups. These vibrations result in H2O (H2O corresponds to CH). They also form nitrogen and hydrogen (NH). The overtones are vibrations that occur at two or three times the fundamental vibration frequency. The absorption intensity of a molecule decreases as the number of overtones increases.
NIR spectroscopy is a useful tool in health and research. It is non-invasive, and can analyze hundreds of samples in a single scan. It can also be used in quality control and cost management. It is useful for studying materials that are not amenable to functional magnetic resonance imaging. In addition, it can be used in the pharmaceutical and agricultural industries.
Cerebral near-infrared spectroscopy is an indirect method of measuring oxygenation and perfusion of the brain. In addition, it can be used to probe the redox state of cytochrome oxidase in the brain. This is a promising non-invasive modality that is being used to monitor patients in hospitals.
In addition, NIR spectroscopy has been used to measure the content of myoglobin in skeletal muscle. It has been used in the medical field for decades. It has also been used in research to study tissue oxygenation in vivo. It has also been used to examine the optical properties of the human body in vivo.
It has also been used in the food industry to determine the chemical composition of pulps. It has also been used to study muscle echo intensity. The success of near-infrared spectroscopy has been achieved through several factors. These factors include a wide spectrum of absorption bands, the capability to absorb multiple constituents at once, and the ability to perform noninvasive analyses.
Fluorescence Spectroscopy
Molecular fluorescence spectroscopy is a relatively inexpensive and sensitive technique that can detect chemical species at very low concentrations. It is widely used to study the structural changes in rigid planar compounds and aromatic molecules. The technique has several advantages over absorption spectroscopy, including the high sensitivity, the ability to analyze the signal in situ, and the ability to measure fluorescence spectra on-line.
Fluorescence spectroscopy is a widely used technique for studying the structural changes caused by changes in temperature. However, it can also be used to detect changes in the composition of a sample. This information can be used to identify critical process states in real time. It is also useful for studying the interaction of different molecules with each other and with the solvent.
For measuring the intensity of a fluorescence signal, it is important to understand how the emission spectrum is constructed. An emission spectrum is a graphical representation of the intensity of the fluorescence signal at a specific excitation wavelength. The wavelength of the excitation light is usually more than the wavelength of the emission light. During the excitation, the fluorophore undergoes energy loss. As a result, energy transfer between the fluorophore and the medium will also contribute to the fluorescence intensity. In addition, fluorescence intensity will be affected by absorption and photodecomposition.
The excitation wavelength can be varied in 5-10 nm increments. The fluorophore's Stokes shift is also important in determining the spectral bandwidth. The narrower the Stokes shift, the less energy is needed to excite the fluorophore to an excited state. This results in a narrower range of SBW.
The light source intensity varies over time, causing distortion in the emission spectra. This can be corrected using n-PLSDA. However, the corrections needed to produce a true machine-independent spectra will vary between experiments.
Common strategies for data reduction include SNV-transformation and Principal Component Analysis. These methods reduce high-dimensional data sets, while preserving important process variables. The optimal number of classes in a feature map is determined by computing the time-dependent variance of the fluorescence intensities.
Chemometric models for fluorescence spectroscopy can help to improve batch-to-batch reproducibility, while improving control and automation. The PLS-method is the most common technique used for chemometric modeling.
Mass Spectrometry
Using mass spectrometry, you can determine the mass-to-charge ratio of ions. You can also determine the molecular weight of sample components. It is also useful in identifying compounds that are unknown.
Mass spectrometry is a technique that is used in many fields of research. It has been used in medicine and for a variety of inorganic and organic chemical analyses. It is commonly used in conjunction with separation techniques.
To determine the mass-to-charge ratio of molecules, a mass spectrometer must ionize the sample. These ions are then measured. The mass-to-charge ratio is a measurement of the number of ions in a sample divided by the number of atoms in the sample. The mass-to-charge ratio is displayed in the form of a logarithmic scale.
The most common types of mass analyzers are ToF, MS and quadrupole. All of these instruments are used in SIMS instrumentation. However, there are other types of mass analyzers as well. These types include ion traps, quadrupole, ESI-quadrupole, and ESI-quadrupole-quadrupole.
Typically, gas chromatography systems use quadrupole or ion trap mass analyzers. However, they can also be used in tandem with other types of instruments. This increases their sensitivity and specificity. Another type of mass analyzer is MALDI-TOF, which is a technique that uses laser beams to scan a sample. The images produced by this technique are label free. This technique is great for studying large tissue sections.
Another type of ionization technique used in mass spectrometry is hydrogen-exchange mass spectrometry. This technique can study the structure of proteins in solution. HX-MS is useful in studying complex, difficult to purify proteins. It is also used to study protein structures that change over time.
There are two primary ion sources. The first is an electrostatic ion source, such as a brown ion source. The other is a plasma source. This source can be used to generate ions of different m/z ratios. The most common ions generated by these sources are Cs + and O 2 + for positively charged ions and Cs - and O - for negatively charged ions.
In addition to ions generated by the primary ion source, the mass spectrometer must also produce ions. This can be done through hard ionization or soft ionization techniques. Soft ionization techniques use small cluster ions.
Applications of Positron Annihilation Spectroscopy
Performing a Positron annihilation spectroscopy analysis involves studying the voids and defects present in a solid. In most cases, this spectroscopy is non-destructive. In fact, it can be used to determine the quality of a component before it is manufactured.
First-lifetime Components
Various applications of Positron annihilation lifetime spectroscopy (PALS) have been made to a wide range of materials. These include living tissues and polymeric solids. However, there are few papers on the topic. Several researchers have reported a limited number of studies on the topic. In some of these studies, only the intensity of the source component of a positron was measured. This approach does not account for the presence of other types of defects that may affect the performance of the irradiated material. For example, vacancy-type defects have been shown to have limited effect on the performance of irradiated materials. Several research studies have also studied positron diffusion in ion-irradiated Fe-based alloys.
In these studies, a total of four x 106 annihilation events were collected. The results showed a clear transition between the displacement damage peak and the displacement damage peak at 600 nm. In addition, a long lifetime component was detected. This component is associated with vacancy-like defects and is produced by adsorption of water molecules at the pores in the material. It is also associated with the formation of o -Ps.
The intensity of the long lifetime component is lower than that of the intermediate lifetime component. This suggests that there are very few thermal vacancies in the material. However, the long lifetime component has a higher intensity when the material is rolled, which is a sign of the presence of high dislocation density. It is also possible that this component is due to the penetration of water molecules into deeper pores. This could explain the unexpected increase in positron diffusion length.
In addition to the effect of surface, the total S parameter is also dependent on the type of defect. In this study, the S parameter of a positron is defined as the sum of the partial S parameters. The partial S parameters correspond to annihilation of free positrons in the medium and positrons trapped at defects. The positronium component of t3 is estimated to be between 0.2 and 0.4%. In addition, the positronium component of t3 depends on the properties of the material, such as the size of pores and surface roughness. In addition, the positronium contribution of t3 may be higher in a polycrystalline Al annealed sample. This component is associated with the formation of o -Ps within the pores.
Various studies have also shown that the intensity of the source component of a sample is related to the electron densities of the surrounding medium. The helium effect has been observed to reduce the intensity of positron trapping at small vacancy clusters. This effect has been observed in Fe-9Cr steel and Fe-9Cr steel ODS variant. However, helium implantation also increases the intensity of the positron mean lifetime.
The S parameter of the positron was also measured by DBS. The average intensity of the source component for the oxide and Al samples was 14% and 12%, respectively. However, the S parameter for the cellulose fiber sheet sample was 32.1 +- 9.9 ps. The S parameter of the cellulose fiber sheet sample was significantly higher in a humid atmosphere than in a dry atmosphere.
Sensitivity
Using positron annihilation spectroscopy (PALS), nanoparticle-embedded substances such as nanoscale thin polymeric films and porous ceramics were studied to determine defect concentrations and the physical structures of interfacial voids. It was also used to study the mobility of self-assembled lipid molecules and molecular packing. The orthopositronium (oPs) lifetime and intensity signatures were sensitive to molecular packing and mobility. It was also used to assess the influence of composition changes on the mobility of the lipid molecules. It was found that oPs formed in minute concentrations and that the mobility of lipid molecules was significantly affected by the composition of the self-assembled nano-particles. In addition, a systematic study using non-ionic micellar systems was also performed.
It was found that positron annihilation induced Auger electron spectroscopy (PALS) was a new surface-sensitive technique that has an exceptional elemental selectivity. It was used to study the surface properties of crystalline and amorphous SiOsub 2 and oxygen-terminated Si-surfaces. A Doppler broadening spectroscopy was also used to study defects in oxides. It was found that there were three distinct defect phases in oxide materials: lacunar-type defects, mesopores and deep potential wells. This information provided insight into the defect characteristics of these materials and their effects on electrical resistivity.
In addition to the study of defect characteristics, it was found that the size of the defected zones could be measured as a function of the depth. The defected zones grew in size with higher pressures applied to the samples. These defected zones had a thickness of 110 mm for 5 bar and 30 mm for 1 bar. In addition, a gradient in density was observed in the SiO2 films. It was also found that the effective oxygen charge was higher in the SiO2 films than in the quartz crystal. This could be due to the difference in W parameters of the two materials.
The role of oxygen vacancies in oxide materials is important to understanding their physical and magnetic properties. In particular, oxygen vacancies affect charge carrier density and paramagnetic properties of diluted magnetic semiconductors. Oxygen vacancies can also play a significant role in high-Tc superconductors. These findings are in accordance with earlier optical studies. It has also been found that the energy profile of annihilation gamma-rays depended on the kind of metal ions used.
It was also found that positron annihilation lifetime spectroscopy (PALS) can be used to study the chemical composition of porous materials. It can provide information on defects produced by charged particles and ions. It has also been used to study the interfacial properties of polymeric nanocomposites. It was also used to study defect induced by irradiation. It was also used to investigate the chemical composition of porous quartz. In addition, it was found that nanodefects could be formed in porous silicium.
It was found that the density of positrons was in open double layers of BiOsub 2. It was also found that there was no significant change in the positron lifetimes through Tsub c. It was also found that the S-parameter decreased with depth. This indicated that the mesopores were connected to the external surface of the zeolite crystals. The intercrystallite regions provided favorable sites for orthopositronium formation.
Applications
Using positron annihilation spectroscopy to study nanostructures and defects has a broad range of applications. These include the investigation of defects induced by irradiation, characterizing defects in nanostructured substances, and studying chemical composition and structure of porous materials. In addition to these applications, positron annihilation specroscopy has been used to study defects in crystalline materials such as polymers and semiconductors. It has been used to study defects caused by charged particles, as well as defects produced by ions.
The most common application of positron annihilation scproscopy is to determine nanoobject size. This is achieved by subtracting annihilation events from the spectrum. This allows direct measurement of thin foils that are widely used in mechanical and electronic applications. However, positron annihilation lifetime spectroscopy presents some limitations. It is important to recognize these limitations when applying positron annihilation lifetime techniques to nanostructured materials. In this article, we will discuss the potential applications of positron annihilation lifetime measurements and demonstrate how it can be used to characterize nanostructured materials.
In this study, nanodefects were measured in porous quartz. Nanodefects in this material have important effects on the physical and chemical properties of this material. In particular, oxygen vacancies have been observed to play a vital role in the properties of the material. Oxygen vacancies can also affect the magnetic properties of diluted magnetic semiconductors. It is therefore important to study the structure of these materials in order to determine the impact of the vacancies. The following study describes the results of positron annihilation studies of nanodefects in porous quartz.
In addition to the effects of irradiation, temperature changes and composition changes were also investigated in this study. In addition, the impact of source correction terms on the positron lifetime spectra was investigated. The results were in accordance with earlier optical studies. The energy profile of the annihilation gamma-rays was found to be similar in all metal acetylacetonates. This was supported by the results from simulations. These simulations were carried out for a single lifetime material and a material with two lifetime components. This was done using the x3-x2-coupling decomposition algorithm, which allows estimation of the free volume of interfacial voids.
Another application of positron annihilation is to study the structure of nanoparticle-embedded substances. This was done using a ionic micellar system, and it was also investigated using a non-ionic micellar system. These systems were studied at different concentrations of surfactant and the results were compared with the results of the micellar system. These studies show that oxygen vacancies can have an important impact on the physical and magnetic properties of porous quartz. These results are in accordance with earlier results and show that this type of study is very useful for examining the irradiation-induced defects in porous materials.
Positron annihilation lifetime spectroscopy (PALS) has been used to characterize nanostructured self-assembled amphiphile systems. Unlike conventional PALS, it is capable of measuring chemical composition in porous materials. In addition, it can be used to characterize defects in nanostructured semiconductors, polymers, and porous materials. Moreover, positron annihilation studies have been proposed as a potential tool for cancer detection.
Video: Positron Annihilation Lifetime Spectroscopy (PALS)
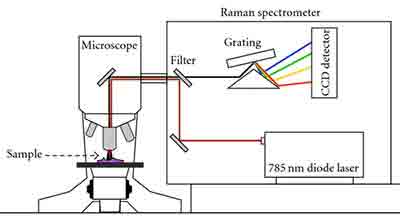
Applications of Raman Micro Spectroscopy
Using the Raman micro spectroscopy technique, we can find out what the chemical composition of a sample is. This is a process that can be used in a non-destructive way. The technique requires only a small amount of preparation and allows us to see the chemistry of a sample.
Surface-enhanced Raman Spectroscopy
Compared to conventional Raman spectroscopy, surface-enhanced Raman spectroscopy (SERS) is a highly sensitive and selective technique. It provides specific information on the properties of molecules by amplifying the Raman scattering signal. This technique has found application in biological and clinical studies, including drug analysis, protein localization, and pathogen detection.
SERS is a vibrational spectroscopic technique, which uses the localized resonance of surface plasmons to generate the Raman scattering signal. The method provides fine molecular fingerprints that allow for direct identification of target analytes. Its sensitivity and selectivity make it suitable for a variety of applications. This technique is non-destructive and is widely used in biomedicine.
Surface enhanced Raman spectroscopy has gained considerable attention over the past four decades. Recent advances have expanded its application from biomedical research to pathogen detection. It is also being used for monitoring chemical reactions in real time. It is highly selective and sensitive, with a high degree of reproducibility.
SERS can be used to perform rapid, low cost assays for detecting pathogens. It can also be used to identify circulating tumor cells and nucleic acids. Unlike conventional Raman spectroscopy, SERS is not sensitive to water. In addition to its high sensitivity, surface enhanced Raman spectroscopy provides fine molecular fingerprints that are useful in many biochemical applications.
There are two main mechanisms for increasing the Raman signal. Chemical contributions contribute a relatively smaller portion of the enhancement, while electromagnetic surface-averaged intensity enhancement factors are the dominant mechanism. The strength of the effect depends on the molecule and matrix.
Giant Chromosomes
Biological research has provided numerous applications of Raman microspectroscopy. The technique allows non-invasive measurements of minute amounts of material. For example, it has been used to study cataractous spots in eye lenses and drug concentrations in cells. The technique can also be applied to liquids, gaseous phases, and subtle complex systems.
Raman microspectroscopy can be used in two modes: Raman direct imaging and Raman line scanning. The former is used for non-invasive measurements and the latter is used for measuring resonance Raman spectra.
In order to perform Raman microspectroscopy, one needs to have two sets of components: a CCD camera and an objective. In addition, a light source is required, such as a krypton-ion laser or an argon-ion laser.
The CCD camera is made by Princeton Instruments Inc. and the objective is a water immersion objective. Both devices were designed for Raman microscopic instrumentation. They allow recording of resonance Raman spectra from 413 nm laser light. The spectra are then registered and the white light image is obtained.
The Chironomus polytene chromosome has a Raman spectrum. The spectrum is characterized by the presence of a dark band in the 1094 cm-1 band. This band is caused by phosphate stretching motion along the DNA. In this region, the presence of a higher concentration of proteins is observed.
The chromatin is organized into histone octamere/DNA complexes and nucleosomes. These two structures are key to the higher order chromatin organization. In addition, the organization is influenced by the activity of non-histone proteins.
Magnesium
Using Raman microspectroscopy, we studied the crystallinity and hydration properties of a magnesium based raw material used for creating white and red painting layers. Spectra of the layers were collected from different latitudes and saturation levels. The resulting Raman spectra were analyzed with infrared emission spectroscopy and high resolution thermogravimetric analysis.
Magnesium stearate was compared with organic and inorganic excipients. The first derivative spectra showed clear distinctions between magnesium stearate and the organic excipients. However, some of the peaks were too far from characteristic water peaks and were therefore less informative. The peaks at 280, 444, and 775 cm -1 were not interesting from an analytical standpoint. In contrast, the peak at 3652 cm -1 corresponded to an A1g O-H stretch vibration in Mg(OH)2.
Three-dimensional crystals were identified from the Raman spectra. The fingerprint of these three peaks, which corresponded to the crystallinity of the bulk, was considered as proof of crystallinity. The intensity of the peak at 3652 cm -1 varied with the magnesium content and was a function of the relative molar ratios of the MgO and MgCl 2 constituent components. In future studies, the use of Raman spectroscopy to identify magnesium stearate will be investigated.
The spectra of magnesium stearate in lubricated calcium phosphate are clearly distinguishable from those of organic and inorganic excipients. In addition to the intensity of the symmetric stretch band at 2848 cm -1, a number of bands are found in the range of 2800 to 3000 cm -1. In addition, a peak at 795 eV is deconvoluted into two maxima. Interestingly, the centers of the two peaks are at 779 eV. The two maxima suggest that the bands may be the result of an antisymmetric stretching vibration of CH 3.
The presence of black filaments was not observed in strongly alkaline solutions. The black areas were associated with the filaments.
Lipid Chains
Various lipids are known to have specific physical properties that can be measured by Raman microspectroscopy. These properties include chain length, unsaturation, viscosity, and packing. The nonpolar nature of lipids gives them a strong Raman cross-section. These physical properties are homeostatically maintained in cells, and are important in their physiological functions.
In this study, a lipid mixture composed of C16:0(d31) and C18:1(d0) was used to investigate the effect of mixing these two fatty acids on spectral transitions. A series of in vitro spectra was generated from the mixture. The spectral transitions of the mixture were compared with those of the single species.
The in vivo spectra of C16:0(d31) show a wider maximum peak at 2100 cm-1. A slight right shift of the peak top is also observed. The spectral transitions of the C16:0(d31) in vivo resemble those reported by previous studies. The spectra show gradual changes in intensity depending on the amount of C16:0(d31) present. This is probably caused by the effect of temperature. The LDs may be acting as a buffer against excess of the gauche/trans ratio.
In the next section, we will investigate the effect of temperature on the in vivo spectral transitions of C16:0(d31). We will also explore the effects of intermolecular interactions. These interactions affect the shape of the Raman spectrum and can affect the structural order of the lipids.
We can predict the compositional information of the human meibum by analyzing the characteristic Raman scattering peaks. These peaks are indicative of the ratio of unsaturated-to-saturated carbon-carbon bonds in algal lipids. The CH stretching band region predominates the Raman spectra of human meibum. The spectra also contain some carotenoid-like bands that may be promising for a spectral feature that can help distinguish donors with normal meibomian gland function from donors with meibomian gland dysfunction.
Ochre
Using Raman microspectroscopy, we studied the micro-residues on ochre pieces at the Sibudu archaeological site. This site is located near the eastern coast of South Africa. The site is composed of 2.7 metres of Middle Stone Age sediments.
The ochre pieces are diverse in composition, morphology, and colour. These results provide information about the natural variability of ochre. In addition, these results suggest that ochre pieces may have been processed into different products.
These results indicate that ochres may have been used to protect leather from bacteria. In addition, ochre powder may have been used as medicine by humans.
Using Raman microspectroscopy, manganese oxides were identified in various ochre samples. These samples had spectra with similar peaks to those found on stone tools. In addition, these samples showed a similar chemical signature to sediment particles.
The samples had a medium hardness and a clayey/silty grain size. The samples also had a red streak, which could have been due to the ochre rubbing against another harder material. The samples also had wear traces that could have come from Middle Stone Age occupations.
Some samples also displayed a high concentration of iron. This was especially the case for Y4-01, a yellow ochre. This concentration was not observed in Y4-07 or Y4-08. The presence of iron in Y4-01, however, did not provide any information about Raman alternatives.
A number of samples contained hematite and goethite residues that may have come from the ochre processing. These compounds were present on the surfaces of the grindstone, and were also found in the sediment.
Al2O3 pellets
Using Raman microspectroscopy, we studied the effect of incipient wetness on the chemical properties of Al2O3 pellets. The purpose of this study is to demonstrate how Raman spectroscopy can be used to quantify and characterize complex multiphase systems. In this study, binary phase samples were investigated, including TiN-MA, TiN-Al2O3, and TiN-MgO*Al2O3. Detailed analysis of the binary phase samples was carried out to understand the relationship between relative Raman peak intensity and phase content. In this study, the relative Raman peak area was determined from the XRF data using Equation 1.
The relative Raman peak area is a measure of the relative content of the phases. Relative Raman peak area is useful for qualitative estimation of the phases. In this study, the relative Raman phase area ratios for Ti2O3-Al2O3 and TiN-MgO*Al2O3 are 416/157 and 157/157, respectively. The coefficient of determination of these ratios is 0.98. The best possible ratio for estimating the phase content is shown in Figure 4.
The use of Raman spectroscopy to evaluate the relative phase content of complex multiphase systems was demonstrated. The spectral properties of individual phases were determined using the PLS regression model. The model was applied to the raw Raman spectral data to establish the relationship between relative Raman peak intensity and the phase content. The resulting model was tested with cross-validation. The model was found to be robust. The model was found to predict phase content in binary samples with root mean square error of prediction (RMSE).
The spectral properties of individual phases were estimated using the PLS regression model. The RMSE was evaluated with cross-validation. The spectral properties of the phases were found to be in good agreement with the PLS regression model.
Video: Basics of Raman Spectroscopy
What Is Positron Annihilation Spectroscopy?
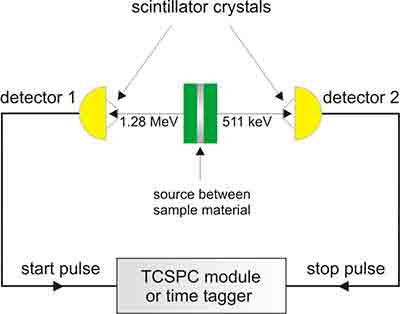
Positron Annihilation Spectroscopy (PAS) is a powerful and non-destructive analytical technique used to study atomic-scale defects in materials. It is based on the annihilation of positrons (antielectrons) with electrons in a material, producing detectable gamma-ray photons. The characteristics of these annihilation events provide information about the material's electronic structure, defects, and porosity.
Principle of PAS
- Positron Generation: Positrons are introduced into the material, typically from a radioactive source like 22^{22}Na or through a positron beam.
- Thermalization and Diffusion: The positrons quickly lose energy and diffuse through the material, where they can become trapped in vacancies, dislocations, or other defects.
- Annihilation: The positron eventually encounters an electron, leading to annihilation and the emission of gamma rays, usually two 511 keV photons in opposite directions.
- Measurement and Analysis:
- Lifetime Spectroscopy (PALS): Measures how long the positron survives before annihilation, giving insights into defect size and concentration.
- Doppler Broadening Spectroscopy (DBS): Examines the energy distribution of annihilation gamma photons, which depends on the local electron density.
- Angular Correlation Spectroscopy (ACAR): Analyzes the angular deviation of emitted gamma photons to infer electronic momentum distribution.
Applications of PAS
- Defect Characterization: Detects vacancies, voids, dislocations, and nanopores in metals, semiconductors, and insulators.
- Thin Films and Coatings: Evaluates porosity and interface properties in thin films, including SiO₂ and other oxide layers.
- Semiconductor Research: Identifies point defects in silicon, gallium arsenide (GaAs), and other materials used in microelectronics.
- Porous Materials: Measures free volume in polymers, zeolites, and porous silicon for applications in filtration and sensors.
- Radiation Damage Studies: Investigates defect formation in materials exposed to radiation, such as nuclear reactor components.
Since you are working on porous silicon and RF thin-film devices, PAS could be useful for characterizing the porosity and defect structures in your materials. Would you like details on how PAS applies specifically to your research?
Subsrates for The Various Types of Spectroscopy
Here are various types of spectroscopy commonly used in scientific research and analysis:
-
Absorption Spectroscopy: Measures the absorption of light by a sample at different wavelengths.
- Ultraviolet-Visible (UV-Vis) Spectroscopy
- Infrared (IR) Spectroscopy
- X-ray Absorption Spectroscopy (XAS)
- Wafer Specs
- Emission Spectroscopy: Measures the light emitted by a sample.
- Flame Emission Spectroscopy (FES)
- Atomic Emission Spectroscopy (AES)
- X-ray Emission Spectroscopy (XES)
- Fluorescence Spectroscopy: Measures the fluorescence emitted by a sample after it has absorbed light.
- Raman Spectroscopy: Measures the scattering of light as it interacts with molecular vibrations.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: Measures the interaction of nuclear spins when placed in a magnetic field.
- Mass Spectrometry (MS): Measures the mass-to-charge ratio of ions to identify and quantify molecules.
- Electron Spin Resonance (ESR) or Electron Paramagnetic Resonance (EPR) Spectroscopy: Measures the magnetic fields associated with unpaired electrons.
- Fourier Transform Infrared (FTIR) Spectroscopy: A type of IR spectroscopy that uses Fourier transform to obtain an infrared spectrum.
- Surface Plasmon Resonance (SPR) Spectroscopy: Measures changes in the refractive index near a sensor surface to study molecular interactions.
- Photoelectron Spectroscopy (PES)
- Ultraviolet Photoelectron Spectroscopy (UPS)
- X-ray Photoelectron Spectroscopy (XPS)
- Circular Dichroism (CD) Spectroscopy: Measures the differential absorption of left- and right-handed circularly polarized light.
- Time-Resolved Spectroscopy: Measures changes in spectroscopic properties over time.
- Mössbauer Spectroscopy: Based on the Mössbauer effect, it measures the resonance absorption of gamma rays.
- Terahertz (THz) Spectroscopy: Measures the interaction of terahertz radiation with a sample.
- Neutron Spectroscopy: Measures the energy distribution of neutrons after interacting with a sample.
- X-ray Diffraction (XRD) Spectroscopy: Measures the diffraction patterns of X-rays passing through a crystalline sample.
- Photoacoustic Spectroscopy: Measures the sound waves produced after a sample absorbs light.
- Laser-Induced Breakdown Spectroscopy (LIBS): Uses a laser pulse to create a plasma and measure the emitted light.
- Rotational Spectroscopy: Measures the rotational transitions of molecules.
- Vibrational Spectroscopy: Focuses on vibrational transitions, including IR and Raman spectroscopy.
These techniques are utilized across various scientific disciplines, including chemistry, physics, biology, and material science, to analyze the composition, structure, and properties of different substances.

