I am a graduate researcher and am looking for a small quantity supply of Lithium Tantalate thin film wafers. Can I speak to someone about pricing? Also, do you have any electrocaloric materials in wafer form?
Lithium Tantalate (LiTaO3)
Lithium tantalate (LiTaO3), also known as ln crystal, is a type of typical  multifunctional crystal. It is a crystalline solid with unique optical properties such as high surface area, high density and high thermal conductivity.
multifunctional crystal. It is a crystalline solid with unique optical properties such as high surface area, high density and high thermal conductivity.
Researcher requested the following quote:
UniversityWafer, Inc. Quoted
LiTaO3 material is a very good electrocaloric material. LT material is a very good electrocaloric material.
LiTaO3 Item #U01-220125-3
76.2mm Z-Cut 0.5mm SAW - Contact us for pricing.
Get your LiTaO2 Quote Today! Or, Buy Online and Save!
LiTaO3 Piezoelecrtric Tranducer
A PhD student requested the following quote:
I am in India and doing my own experiments and looking for helping good people like you as I have to invest on my experiments. Now I want highest quality of Lithium Tantalate crystal of size say 10 cm X 3 cm. If you have different size quote me for that nearby size. You give me your specs of the item and let me know its price so that I can decide the quantity to buy from you as per available funds with me here. I may need 2 pcs of 10cmX4cmX2cm.
To see whether it helps me or not. Later quantities could be decided. Please see that highest efficiency material to use in ferroelectricity Lithium tantalate is needed. Let me know the solar cell wafers that you manufacture details including efficiency and price.
We can join to avert pollution and global warming through my experimental work. Please understand this and let me know your opinion. Lithium tantalate should be of the type used in Low thermal fusion type.
Reference #242930 for specs and pricing.
Lithium Tantalate Wafer (LiTaO3)
Lithium tantalate (LiTaO3) piezoelectric and pyroelectric properties are great for nonlinear optics, passive infrared sensors, terahertz generation & motion detectors, surface acoustic wave applications.
LiTaO3 Pyrotechnic Detector
The LITAO3 (Lithium Tantalate) pyroelectric detector is one of the most widely used and simplest pyroelectric detectors. Its properties are as follows: density 7.5 G CM-3, specific heat capacity 0.43 J K-1, a thickness of 0.2 mm, and a diameter of 10 mm. The detectivity of this material is rated at DT=10 MS.
A LiTaO3 pyroelectric detector is an excellent candidate for detecting broad-spectrum radiation. The underlying principle is simple: heating the crystal to a certain temperature results in the generation of a charge, which is then connected to an external circuit. The resulting current is proportional to the rate at which the crystal temperature changes. This fundamental approach to enhancing the sensitivity of a pyroelectric detector consists of optimizing the materials used and improving their performance.
The lithium tantalate crystal reacts with light rays and creates an opposite surface charge. The opposite surface charge equalises and visible sparks are generated. The output voltage and pyroelectric current are then calculated. The maximum output voltage is then calculated from this calculation. The detection range of a pyroelectric detector depends on the temperature. Typically, the temperature is about 20 degrees Celsius. The input radiation pulse has a duration of ten milliseconds, and the whole spectrum of incident radiation is absorbed.
The pyroelectric material lithium tantalate is one of the most widely used pyroelectric materials. Its thermal conductivity is high and it is often used as a power monitor in pulsed laser systems. A lithium tantalate pyroelectric detector is made up of a single-crystalline lithium tantalate. This crystalline material has an excellent long-term stability of the signal voltage
Lithium Tantalate Substrate Applications
There are a wide variety of applications for lithium tantalate substrate,  but none is more interesting than its use as a piezoelectric material. This material has a controlled volume resistivity and sufficient piezoelectric properties to be used in a variety of devices. For example, a capacitor made of lithium tantalate can produce an electric current that can be used for a wide range of high-voltage applications.
but none is more interesting than its use as a piezoelectric material. This material has a controlled volume resistivity and sufficient piezoelectric properties to be used in a variety of devices. For example, a capacitor made of lithium tantalate can produce an electric current that can be used for a wide range of high-voltage applications.
The 36deg and 42deg cuts of lithium tantalate have seen widespread application in the mobile phone handset industry. The insertion loss is optimized with the 42deg cut. In addition, the 112deg rotational cut has shown great promise for lower bandwidth and higher selection filters. This material is also being used to replace quartz in optical components and is expected to fill the gap between Quartz and the 36/42deg models.
Another promising market is the telecommunication industry, which is driven by the need for speed and connectivity. 5G networks will continue to grow and drive sales of lithium tantalate crystal. Further, governments are adopting the technology and digitalizing cities through various projects like Smart Cities. These technologies will continue to drive the demand for lithium tantalate substrates. These technologies are becoming commonplace and will continue to drive growth in this industry.
In the mobile phone industry, lithium tantalate is used in the production of a number of different semiconductors. The 36deg and 42deg cut of lithium tantalate are particularly popular in this field. Their combination of piezoelectric and electro-optical properties will allow them to be used in a variety of nonlinear optical devices. With these unique properties, lithium tantalate is the ideal material for use in many diverse fields.
While the use of lithium tantalate in a number of different fields is essential, its most popular application is in the manufacturing of cellular phone handsets. The 36deg cut of lithium tantalate is often used in this market. However, LT has a high melting point and is commonly used in telecommunications. In addition to this, the material is also used in the fabrication of wireless devices and in the fabrication of solar cells.
Because of the unique properties of lithium tantalate, it is widely used in a number of different fields. It is also found in a variety of different acoustic devices. It can also be used in piezoelectric sensors. Despite its unique properties, it is currently only used in electro-optical devices. But other areas where it is used include: telecommunications, acoustic wave generation, and energy harvesting.
Among its primary uses, lithium tantalate is used in the manufacture of telecommunications and optical devices. While the chemical stability and high mechanical resistance of the lithium tantalate wafer makes it useful for telecommunications equipment, its high optical damage threshold and good electrical conductivity make it useful in the manufacture of a wide variety of consumer electronics. These products are used in cell phones as display screens and as interdigital transducers.
Another major application for lithium tantalate is in the telecommunications industry. The telecommunications industry is a driver for the demand for lithium tantalate crystals. The Internet of Things (IoT) is a key market for the material, and as the Internet of Things becomes more prevalent, the lithium tantalate crystal will play a major role in supporting these technologies. In addition, the government is actively digitalizing cities under the Smart City project.
Moreover, lithium tantalate is used in a variety of other industries as a piezoelectric substrate. Its wide range of properties makes it an ideal choice for a wide variety of electronic devices. Its main uses are in the telecommunications industry, including telecommunications equipment and mobile phones. The semiconductor industry is also a major driver for the lithium tantalate crystal market.
The LT substrate is an excellent choice for making optical devices and semiconductors. Its high light transmittance properties are particularly useful in photolithography. The LT substrate is especially useful in optical devices for the IoT. In fact, it can be used in the fabrication of various types of electronic components, such as chips and other electronics. As a result, the market for lithium tantalate crystals is increasing.
Lithium tantalate (LiTaO3) paired with gallium nitride (GaN) substrates is a notable combination in the field of electronics and photonics. Here's how they work together:
How Does Lithium Tantalate Work with Gallium Nitride Substrates?
-
Material Properties: Lithium tantalate is a ferroelectric material known for its excellent piezoelectric and pyroelectric properties. Gallium nitride, on the other hand, is a semiconductor known for its high thermal conductivity, high electron mobility, and wide bandgap. The combination of these two materials leverages the strengths of both.
-
Lattice Matching: One of the critical aspects of using LiTaO3 with GaN is lattice matching. This means the crystal structures of both materials need to align well to minimize defects at the interface. A good lattice match results in better electronic and optical properties of the combined material.
-
Epitaxial Growth: GaN can be grown epitaxially on LiTaO3 substrates. This process involves depositing GaN in a crystallographically oriented manner on LiTaO3. Epitaxial growth is crucial for high-quality crystal formation, which is vital for electronic and photonic applications.
-
Applications in Optoelectronics: The combination is particularly useful in optoelectronic applications. For instance, GaN on LiTaO3 can be used to create high-frequency piezoelectric resonators and filters, which are essential components in RF (Radio Frequency) and microwave communication systems.
-
Thermal and Mechanical Stability: GaN has a high thermal stability, which, when combined with the piezoelectric properties of LiTaO3, makes the composite material suitable for high-temperature and high-power applications.
-
Electrical Properties: The interface between LiTaO3 and GaN can exhibit unique electrical properties, like high electron mobility, which is beneficial for high-speed electronic devices.
-
Challenges: Despite the advantages, there are challenges in combining these two materials. Mismatch in thermal expansion coefficients and potential chemical reactions at the interface need to be managed for optimal performance.
In summary, the combination of lithium tantalate and gallium nitride substrates offers promising prospects in advanced electronic and optoelectronic devices, leveraging the unique properties of both materials. However, careful consideration of material compatibility and epitaxial growth conditions is crucial for achieving the desired performance in practical applications.
LiTaO3 for Pyroelectric Applications
A materials scientist requested a quote for the following:
We manufacture pyroelectric flame dedectors. So we need poled, Z cut, 4 inch, 750 nm LiTaO3 wafer for pyroelectric applications. We are searching for favorable company that fit our pyroelectric applications as if it is found we are going to purchase 1k to 100k pieces. Could you please tell us your options about poled LiTaO3 or a commercial pyroelectric wafers to initially order a few. Look forward to hear from you.
Reference # 252296 for specs and pricing.
Lithium Tantalate (LiTaO3) Wafers for Research and Production
Lithium Tantalate (LiTaO3) in stock. Best prices in small quantities for research and production.
It is a multifunction crystal material with chemical aNd mechanical stability.
Because LiTaO3 crystals have a high optical damage threshold, they are often found in the following:
- Interdigital transducers
- EO
- Pyroelectric IR detectors
- Elecrocaloric Devices
Single-crystal Lithium Tantalate Thin Film
Free Standing Ultra Thin & Ultra Flat Wafers |
|||
|---|---|---|---|
| Diameter | 76.2mm, 100mm | Ori | X, Y, Z, AT etc. |
| Thickness | 10-60 micron | Material | LN, LT, Si, Quartz etc |
| Surface | Double or Single Side Polished | ||
Lithium Tantalate Crystals and Inventory
Below are just some of th LiTaO3 Crystal that we have in stock. Please let us know if you can use or if you need another spec quoted.
| Surface Finish | Outer Diameter | Thickness | Brand/Grade | Top side Ra | Backside Ra |
| DSL | 14+/-0.1mm | 0.5+/-0.03mm | Grade I | GC#2000 | GC#2000 |
| DSP | 14+/-0.1mm | 0.5+/-0.03mm | Grade I | < 1 nm | < 1 nm |
| DSL | 10*20+/-0.1mm | 0.5+/-0.03mm | Grade I | GC#2000 | GC#2000 |
| DSP | 10*20+/-0.1mm | 0.5+/-0.03mm | Grade I | < 1 nm | < 1 nm |
| DSL | 5*5+/-0.1mm | 0.5+/-0.03mm | Grade I | GC#2000 | GC#2000 |
| DSP | 5*5+/-0.1mm | 0.5+/-0.03mm | Grade I | < 1 nm | < 1 nm |
According to a recent research report, the global market for lithium tantalate crystals is gaining in luster as we approach the end of the first half of 2017 and the beginning of 2018. It is at this point that we announce the launch of a new lithium tantalate wafer product line, Lithium Tantalates (LTA). [Sources: 1, 4]
The growth of the market is due to the growing applications of lithium tantalum crystals in a variety of applications. This growth will unfold in the acoustic wave devices in which they are used to manufacture electrical components. The increasing use of these technologies in high-performance electronic devices is driving the industry's demand to a high degree. Increasing applications in medical applications such as medical devices and medical imaging are expected to drive the market for lithium tantalate wafers. [Sources: 1, 3, 6, 8]
The methods used in this invention are of industrial advantage, as the above-mentioned lithium tantalate crystals can be obtained in a relatively short time. [Sources: 7]
It may be interesting, however, that although the first synthesis of lithium tantalate took place in Russia, Asia-Pacific still holds a huge share of the market. The growing demand for lithium-niobate crystals, especially in China and India, will give a boost to the growing lithium-tantalate crystal industry. It is expected to boost the market for lithium tantalates during the forecast period. However, most manufacturers develop lithium niobate crystals in the United States, thus replacing the low threat. [Sources: 1, 3]
To produce a SAW device with lithium tantalate crystals, it must be heated in a high-temperature environment, as is used in production facilities today. It is also preferable to use wafers that have undergone lapping or at least slicing, or one side of which has undergone mirror polishing to polarize the lithium tantalate crystal into a single one. It is possible to process lithium niobate, which can be used for the manufacture of integrated circuits and other electronic devices. In addition, lithium tantalates can also be used for the development and manufacture of an integrated circuit with a wafer. [Sources: 0, 7, 9]
According to the present invention, a single polarized lithium tantalate may be so thin that its electrical conductivity is 1a10a12 c / a1A / cma1 or more and the surface charge which generates pyroelectric properties decays quickly. In addition, the charge can also be generated by applying a charge from a surface layer of lithium-niobate to the top of the wafer, with the lithium-tantalate crystal applied. When the electrical conductivities of wafers are more uniform, the lithium tantalate crystals can still be thin enough that the surface charges generated by the pyroelectric properties decay quickly, but not too thin, so that they do not cause any loss of electrical energy. [Sources: 7]
In this case, the reduced atmospheric temperature is 610Adeg C or less, and the surface resistance to warming to 500AdeG C is so high that even lithium-niobate crystals are used, which are said to have a faster reaction rate. This is attributed to the fact that both lithium tantalate and lithium niobates have very high coefficients of thermal expansion. [Sources: 2, 7]
The piezoelectric properties of lithium tantalate crystals are an essential feature when used in SAW devices. Compared to quartz, they have a large electromechanical coupling ratio of 1: 1.5, which has a very high surface-to-surface connection. This shows that they are suitable for this purpose, as the piezoelectrode reaction and piezoelectric properties are derived from the crystal structure of these crystals and are required for the sawing tool. [Sources: 1, 7]
After the single- and polarized lithium tantalate crystals are cut with a saw wire, they undergo the lapping process, resulting in a double-sided, lapped wafer with a thickness of 0.4 mm. This confirms that we have a lithium tantalate wafer (LT - WAFer) that is stacked and a silicon-in-a-silicon wathor that is a load-bearing wafer, both of which have the same surface-to-surface ratio, namely 1: 1.5. [Sources: 2, 7]
We investigated how the variation of the passive lithium permeability by bicarbonate affects the transport of sodium - driven lithium meters. Our results support the hypothesis that this is due to the presence of sodium in the lithium tantalate wafers and not to a change in lithium counter transport capacity. [Sources: 5]
First, the lithium-tantalate polycrystal, which is produced by weighing and mixing lithium carbonate and tantalum entoxide, is placed in a crucible made of precious metals such as iridium. [Sources: 7]
When the temperature is 30 Adeg C or less, the wafers are removed from the iridium crucible into a stainless steel container, which allows them to be stacked on top of each other and the lithium tantalate crystals to undergo the reduction process. It is supplied with lithium carbonate and tantalum entoxide, which is weighed and mixed with a mixture of lithium tantalate polycrystals and other precious metals. [Sources: 7]
Sources:
[0]: https://worldwidescience.org/topicpages/n/niobate+lithium+tantalate.html
[1]: https://www.transparencymarketresearch.com/lithium-tantalate-crystal-market.html
[2]: https://www.freepatentsonline.com/y2019/0097596.html
[3]: https://www.prnewswire.com/news-releases/the-global-lithium-tantalate-wafers-market-size-is-expected-to-reach-usd-2-87-billion-by-2024--300755464.html
[4]: https://www.yumpu.com/en/document/view/38377275/lithium-tantalate-sawyer-technical-materials-llc
[5]: https://www.science.gov/topicpages/l/lithium+tantalate+litao3
[6]: https://www.analystviewmarketinsights.com/report-highlight-lithium-tantalate-wafers-market/
[7]: https://google.com/patents/US20050066879
[8]: https://thekmcollege.com/12583/737
[9]: http://www.discousa.com/eg/news/press/20020806.html
What is The Electrocaloric Cooling Effect (ECE)
The EC effect refers to a change in temperature caused by an electric current applied to a dielectric material.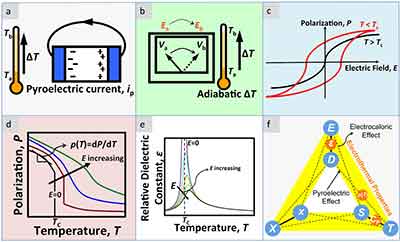 This effect is driven by the quest for new environmentally friendly and energy efficient cooling technologies. The bottleneck of current EC cooling technologies is too small a change in temperature. Research on EC cooling technologies has been focused on the theoretical understanding of the EC and high-performance EC materials. This work highlights the potential for combining these two cooling technologies.
This effect is driven by the quest for new environmentally friendly and energy efficient cooling technologies. The bottleneck of current EC cooling technologies is too small a change in temperature. Research on EC cooling technologies has been focused on the theoretical understanding of the EC and high-performance EC materials. This work highlights the potential for combining these two cooling technologies.
The electrocaloric effect is similar to the photoelectric effect, but occurs in a completely different way. When an electric field is applied to a polar dielectric, the electrocaloric effect causes the material to undergo a change in temperature. This change is analogous to the changes in temperature that occur when a material is stretched or compressed. The EC effect results from the relative movement of ions in the material's structure and their relative order.
The EC effect is a form of adiabatic cooling, and is possible to make electrodes with a thickness of only 0.2 mm. This material is capable of exceeding four-seven degrees Celsius, which is higher than the temperature of the surrounding air. However, this technology is still in the development stages, and further improvements in electrical insulation would be necessary to make it practical for everyday use. There are many advantages to electrocaloric cooling and it has a bright future in many fields.
The development of a new refrigerator has been a priority in the scientific community since the 1997 Kyoto Protocol. But the question remains, "Can we actually use an electrocaloric material in a refrigerator?" The answer is yes, and we may soon have a refrigerator that is environmentally friendly. It's still a work in progress and it will take many years before it becomes practical. It's important to remember that there are many challenges in making a good electrocaloric material.
The electrocaloric effect is similar to the photoelectric effect but differs in its mechanism. The electrocaloric effect is an electrical field applied to a polar dielectric material. This leads to changes in the material's temperature, which is analogous to a change in entropy. As a result, the ECE is a very promising technology. It can be made in thin films, which can be used in electronics and cooling systems.
An electrocaloric material is heated by an electric field. In order to induce the giant DT, it must be placed in a large magnetic field. To induce this effect in a magnetocaloric material, a magnetic field is required. Consequently, it's difficult to use an electrocaloric material in a refrigerator. It is a viable solution for cooling, but this is still a work in progress.
The electrocaloric effect is the opposite of the photoelectric effect. When a polar dielectric material is subjected to an electric field, it will change its temperature. This effect is analogous to the changes in temperature that occur when a material is compressed or stretched. The relative movement of the ions in the structure of an electrocaloric material will change the polarization entropy and the electrical polarization of the material.
The electrocaloric effect is the opposite of the photoelectric effect and is commonly used for refrigeration. It is similar to the thermoelectric effect, but a polar dielectric material has no dielectric property, but it can be cooled with water. This is an excellent example of a low-carbon refrigerator. Moreover, electrocaloric materials are compatible with a wide range of other materials. In addition to the cooling effect, they are also useful for the heating of a variety of different electronic components.
The electrocaloric effect is the opposite of the photoelectric effect. This effect is the result of an electric field applied to a polar dielectric material. This change in temperature is similar to that which occurs when a material is compressed or stretched. The change in temperature is due to the relative movement of ions in the structure. This is a very promising method of cooling a device. Its reversible properties make it a suitable choice for many applications.
Video on the Electrocaloric Effect
Are Lithium Tantalate Thin Film Wafers Good for Fabricating Electrocaloric Materials?
A graduate researchers requested a quote for the following:
I am looking for a small quantity supply of Lithium Tantalate thin film wafers. Can I speak to someone about pricing? Also, do you have any electrocaloric materials in wafer form? I am curious how much it would cost for the 76.2mm diameter ultra flat 60micron wafer as well as a 0.5mm thick one in roughly the same diameter. If you can't do around the same diameter smaller is better, such as around 20mm.
Right now I am looking for about half a dozen or less.
As for the Ori I am not sure. This is the first time for me to buy crystals like this so what options do you have? I will be using the wafer for a sensing application in which the broad sides of the wafer will need to collect the pyroelectric charge.
Obviously, the LT wafer with SSP finish will have good pyroelectric properties but your email suggests it will have good electrocaloric properties as well. Is that true? Do you have a material that has better electrocaloric properties?
I need to run simulations first on those parameters before I purchase. I will be buying something from you soon. I need to determine if the LN or the LT is better suited for my design as well as inputting the real dimensions from your product.
Do you happen to have epsilon and loss tangent data on your LN and LT? Those values both change depending on the frequency, but anything around the 214–400 THz / 0.75 -1.4 um range would be great.
UniversityWafer, Inc. Answered:
We do have some Z-cut such LT wafers in stock and please see the quotation sheet as below:
| Pol | Dia | Ori | Thick | Brand/Grade |
| SSP | 76.2+/-0.2mm | Z-cut | 0.5+/-0.03mm | SAW |
Meanwhile, LT material is a very good electrocaloric material!
Reference #264423 for specs and pricing.
Z-Cut Lithium Tantalate Crystal Substrates Lattice Matching to Fabricate High Performance Gallium Nitride Substrates
A scientist researching manufacturing of gallium nitride (GaN) membranes for efficient thermal management requested the following quote:
Could you please let me know price as follows ? LiTaO3 Lithium Tantalate Crystal Substrates and Wafers, Z-cut, 6 inch diameter, SSP, Qty 5 ea 4, 6, 8 inch Undoped Silicon Germanium (SiGe) Epitaxial Wafer on Silicon (Si), Qty 5 ea 6, 8 inch Silicon Carbide Wafers 4H-SiC Semi-Insulating, MPD grade, Qty 5 ea
UniversityWafer, Inc. Quoted:
The 6" LiTaO3 Ingot with Z-cut is very very short , now we have no stock , it takes about two months for growing and processing, could you consider the other Ori 42Y-cut (normal Ori) ? here is quotation for your refer , the lead time is 2-3weeks
| Dia | Ori | Thick | Pol | Primary Flat | Material | Brand /Grade | Warp | Bow | TTV | Top side Ra |
| 150+/-0.2mm | 42° Y-Cut | 0.35+/-0.03mm | SSP | 47.5±1mm to +X | LiTaO3 | SAW | <50um | <50um | <10um | <1nm |
Reference #269664 for specs and pricing.
LiTaO3 Substrates to Fabricate Photopyroelecrtric Sensors
A optoelectroncs engineer requested the following quote:
I would like to know the price of lithium tantalate crstal wafer for photopyroelectric sensor applications. It should be between 100- 400 micrometer in thickness.
I would like to know the price of LiTaO3 Z-cut diam 10mm x 200 micron thick uncoated. Our purpose is to use in laser-based photopyroelectric spectroscopy. It is used as a sensor by radiating laser on it.
UniversityWafer, Inc. Quoted:
| Pol | Dia | Ori | Thick | Material | Brand/Grade | Top side Ra | Backside Ra | S/D |
| DSP | 10+/-0.2mm | Z-cut | 0.2+/-0.025mm | Lithium Tantalate | Optical | <1nm | <1nm | 40/20 |
Reference #270244 for specs and pricing.
50.8mm Z-Cut LiTaO3 Substrates for Research
A PhD Technology Development Manager RF requested the following quote:
I’m looking for sources for lithium tantalate wafers, z-cut, of various thicknesses. Currently we’re looking at thickness between 0.5mm and 5mm, 1” or 2” diameter. Once we settle on a thickness I think we’d be looking at 100-500 wafers at this time. This is a new application area for us, so I’m not 100% settled on what other specs we need, e.g., polish, grade, etc.
UniversityWafer, Inc. Quoted
| Pol | Dia | Ori | Thic | Material | Brand/Grade | Top side Ra | Backside Ra | S/D |
| DSP | 50.8+/-0.2mm | Z-cut | 0.5+/-0.03mm | Lithium Tantalate | SAW | <1nm | <1nm | 40/20 |
Reference #275723 for specs and pricing.
