Non-Irradiated Sapphire Wafer for Research & Production
Non-Irradiated Sapphire Wafer
In the past, many sapphires were blue and were used in the gemstone and jewelry industry. The synthesis of blue juices came about after chemical analyses by Sapphire Verneuil suggested that iron and titanium were the cause of the blue colour. [Sources: 4, 10]
Due to this behaviour, the silicate glass was chosen as the upper substrate instead of the sapphire glass due to its formability. The optical sapphire fibres are guided through a furnace and the spheres and silicon fusions are outside a heated zone. [Sources: 7, 9]
Get Your Quote FAST!
Non-Irradiated Sapphire Wafer
The following is an interaction between UniversityWafer, Inc and a researcher.
Researcher
I have already purchased item number 1306. Can you please tell me what is C to M-plane means? I am wondering to know the crystal orientation in this sapphire wafer.
Sapphire Wafer Item #1306 - 50.8mm 430um SSP C to M-plane by 0.2±0.1 deg off.
C to M-plane means: C-Plane(0001) off-axis towards to M-axis
UniversityWafer, Inc.
Yes,crystal orientation in this sapphire wafer C-plane,small mis-cut angle @ 0.2deg,of which is a industrial standard C-plane sapphire.
Researcher
One more question, I have irradiated this wafer and I want to study crystal orientation after irradiation then compare the result with non-irradiated wafer. Did you have any data for X-Ray Diffraction (XRD) related study for this non-irradiated sapphire wafer? Thanks you so much.
UniversityWafer, Inc.
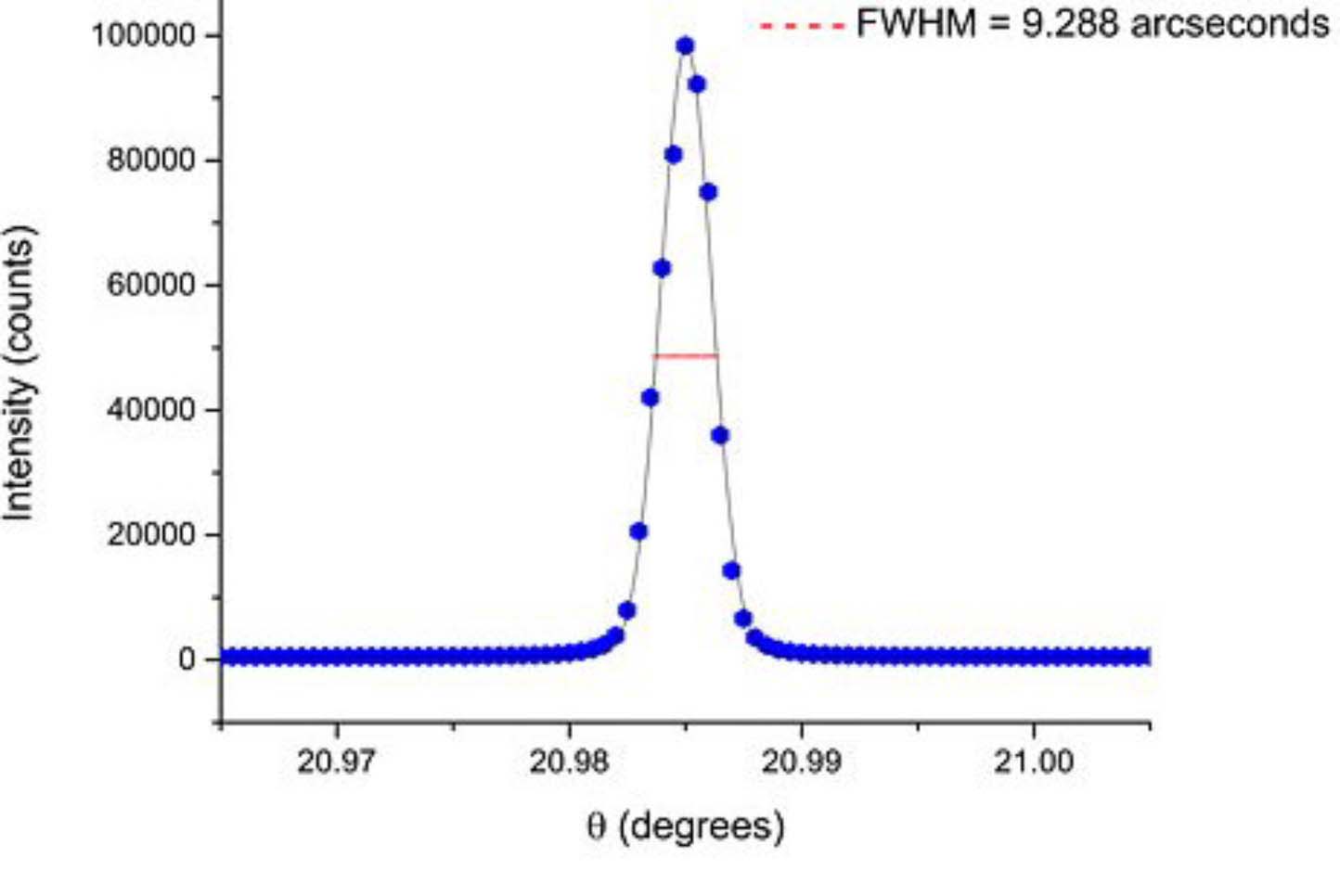
The surface of the sapphire substrate is subjected to plasma activation and bonded at 200Adeg C. Surface and substrate are bonded with 350AdeG C and subjected to ion beam activation treatment. A 3SIO2 coated sapphire wafer and a molten silica wafer are cut into 10x10 mm2 pieces. In addition, the surfaces of both Sapshire substrates and substrates are bonded with 200ADeC and subjected to plasma activation treatment. By bonding them together at 350ADesC, treating them for ion beam activation treatments and connecting them to 400ADegC. [Sources: 8, 9]
The crystalline quality of the sapphire wafer is not affected by the use of activation treatment with non-ionic beams. The incident laser beam from the elevator process carries the resulting damage in an energy density that makes it possible to transfer it to an area of 10x10 mm2 in the surface through the elevator process. [Sources: 0, 11]
Crystallization is favoured and supported by several reasons at the interface between silica and glass. The molten silicon silicon has low thermal conductivity, which is also explained by the high temperature of the sapphire wafer in the presence of a high concentration of carbon dioxide. [Sources: 9]
In addition, the effect of ion-irradiated sapphire crystals on the surface of the wafer is related to light scattering on the crystal surface. As the laser light from the lift passes through the sapphire wafers, damage or dirt in the surfaces of some of them can create shadows, causing defects in the lift interface. The GaN layer can also be broken or broken by the limited ablation of a laser beam with a high concentration of carbon dioxide, which can lead to the formation of shadows. In addition, a small amount of radiation from an ion - irradiated saffron crystal - can also lead to shading in some parts. [Sources: 0, 2, 12]
The formation of crystallographic defects is also facilitated by the high concentration of carbon dioxide in the GaN layer of the wafer, which can also contribute to the formation of shadows on the surface of sapphire wafers. [Sources: 3]
The sapphire, on the other hand, acts as a heat spreader, and the heat generated is dissipated through the stack of its substrate. If the laser beam is carried out according to this setting, it is possible to evaporate the heat from the surface of the wafer by means of laser beams at wavelengths easily absorbed by the sapphires and forming fine grooves [101] in the surfaces of their substrates [101]. A combination of high temperature and low pressure lasers (e.g. 1,2) is used. [Sources: 0, 6, 9]
This is because it is possible to crack the sapphire substrate without greatly reducing its thickness. Cu, which has a high thermal and electrical conductivity as well as a high thermal efficiency [101]. [Sources: 0, 6]
This selective absorption allows laser light to pass through the sapphire and remove the GaN, which leads to separation. Because the spike annealing occurs extremely quickly with increasing temperature, the silicon becomes hotter than the spheres earlier, although the irradiation does not heat them up in the wavelength band. This means that 248 nm radiation is absorbed into the SaN better than out of it, and the actual temperature is less than half the temperature of the thermocouple when infrared light passes through a transparent saffir. The thermal conductivity of a silicon wafer with a radiation-free wafer was measured at 8200C. [Sources: 1, 2, 8]
Al installation in the sapphire substrate, otherwise it would inhibit the crystallization of BTS by laser radiation, as already shown [31]. Since the nitride mass crystals used in practice have not yet been produced, it is inevitable that nitrite crystal films will grow epitaxially on sapphire [sic] substrates due to their high thermal conductivity. [Sources: 6, 9]
GaN decays into gallium and nitrogen, and the crystallographic defects in silicon are caused by the reaction between the sapphire substrate and the silicon film. As the interference-based OFDR sensor technology fails in normal sapphire fibers, it can be turned into a silicon wafer with its large core and crystalline structure. Demand for high-performance, cost-effective and high-performance silicon substrates is growing rapidly [31]. [Sources: 3, 5, 6]
When a sapphire wafer with silicon core and silicon film is formed using the conventional SPE process, the irradiation device degrades quickly. When the device is exposed to ionizing radiation, charges accumulate in the silicon, and when it is irradiated, its performance deteriorates. [Sources: 3]
Sources:
[0]: https://google.com/patents/US7241667?cl=en
[1]: https://www.google.com.gh/patents/WO2009051836A1?cl=en
[2]: https://www.freepatentsonline.com/8986497.html
[3]: https://patents.google.com/patent/US4775641A/en
[4]: https://en.wikipedia.org/wiki/Sapphire
[5]: https://patentswarm.com/patents/US10436978B2
[6]: http://www.google.ch/patents/US6411636
[7]: https://uspto.report/patent/app/20200003949
[8]: https://www.google.com.pg/patents/US8748294
[9]: https://kundoc.com/download/laser-welding-of-fused-silica-glass-with-sapphire-using-a-non-stoichiometric-fre_5aebab48d64ab271a2a08c8a.html
[10]: https://geology.com/gemstones/fancy-sapphire/
[11]: http://dx.doi.org/10.1021/acsomega.0c01824
[12]: https://www.science.gov/topicpages/r/r-plane+sapphire+substrate
