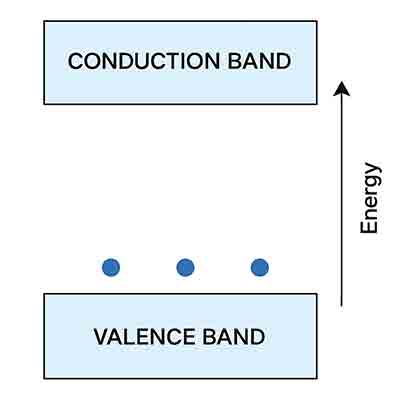
I need the effective density of states near the valence band maximum/conduction band minimum for the n-type, p-type, and intrinsic Si wafer. Could you offer me this information?
What Are The Density of States Near The Valence Band?
A PhD candidate requested a quote for the following:
Question:
Answer:
The effective density of states near the valence band maximum (for p-type) and conduction band minimum (for n-type) in silicon (Si) wafers are important parameters in semiconductor physics. For an intrinsic (undoped) Si wafer, both n-type and p-type properties are relevant. The values depend on the effective mass of the charge carriers (electrons and holes) and the temperature.
The effective density of states in the conduction band (N_C) and valence band (N_V) for silicon at room temperature (around 300 K) are typically given as:
- N_C (conduction band, relevant for n-type Si): Approximately 2.8 x 10^19 cm^-3
- N_V (valence band, relevant for p-type Si): Approximately 1.04 x 10^19 cm^-3
For an intrinsic Si wafer, both N_C and N_V are relevant, and the intrinsic carrier concentration (n_i) can be calculated using these values along with the bandgap energy of silicon and the Boltzmann constant.
Please note that these values are approximate and can vary slightly based on the specific conditions, such as temperature and doping levels. For precise calculations or for conditions significantly different from room temperature, it's recommended to use more specific data or formulas that take into account these variations.
Reference #250233 for more or click here!
Get Your Quote FAST! Or, Buy Online and Start Researching Today!
What is the Difference Between Conduction Band and Valance Band?
The conduction band and valence band are key concepts in the field of solid-state physics, particularly in the study of semiconductors and insulators. Here's the difference between them:
-
Valence Band:
- The valence band is the highest range of electron energies in a solid where electrons are normally present at absolute zero temperature.
- It consists of electrons that are bound to atoms and participate in the formation of chemical bonds.
- These electrons are responsible for the electrical properties of the material in its ground state.
- When electrons are in the valence band, the material behaves as an insulator or a
 semiconductor, depending on the energy gap between the valence band and the conduction band.
semiconductor, depending on the energy gap between the valence band and the conduction band.
-
Conduction Band:
- The conduction band is the range of electron energies higher than the valence band, where free electrons can exist.
- These electrons are not bound to atoms and can move freely through the material, contributing to electrical conductivity.
- In conductors, the conduction band overlaps with the valence band, allowing electrons to move freely even at low energies. In semiconductors and insulators, the conduction band is separated from the valence band by an energy gap.
- When electrons gain enough energy (e.g., through thermal excitation or photon absorption) to jump from the valence band to the conduction band, they contribute to electrical conduction.
The key difference lies in their role in electrical conductivity: electrons in the valence band are involved in bonding and do not contribute significantly to conductivity under normal conditions, whereas electrons in the conduction band are free to move and contribute to the electrical conductivity of the material. The energy gap between these two bands determines the electrical nature of the material (conductor, semiconductor, or insulator).
Why is Valence Important in Semiconductors?
Valence is important in semiconductors because it determines how atoms bond and how electrons behave—both of which directly affect a material's electrical properties.
Here’s why it matters:
🧪 1. Valence Electrons Determine Bonding
-
Semiconductors like silicon (Si) have 4 valence electrons.
-
These electrons form covalent bonds with neighboring atoms in the crystal lattice.
-
A stable, tetrahedral structure is created, which is crucial for the material's semiconducting properties.
⚡ 2. Electrical Conductivity Depends on Valence Band
-
In solid-state physics, the valence band is the highest range of electron energies where electrons are normally present at absolute zero temperature.
-
Above it is the conduction band—electrons must gain energy to jump from the valence band to the conduction band to conduct electricity.
-
The band gap between these bands is what defines a material as a conductor, semiconductor, or insulator.
🧬 3. Doping Relies on Valence
-
Doping modifies electrical properties by introducing atoms with different numbers of valence electrons:
-
Phosphorus (5 valence electrons) → donates extra electrons → n-type semiconductor.
-
Boron (3 valence electrons) → creates "holes" → p-type semiconductor.
-
-
This manipulation allows precise control of conductivity in devices like transistors and diodes.
🏭 4. Device Function Depends on Valence Behavior
-
The switching and amplifying behavior in transistors, photovoltaic effects in solar cells, and light emission in LEDs are all driven by electron transitions involving the valence band.
In short:
Valence electrons govern how atoms interact and how charge carriers are created and move—making them central to how semiconductors function.
Valance vs Valence
In the context of semiconductors, "valence" and "valance" are often confused—but only "valence" is correct and relevant to the science. Here's the difference:
✅ Valence (Correct Term in Semiconductors)
-
Definition: The number of electrons in the outermost shell (valence shell) of an atom.
-
Why it matters in semiconductors:
-
Silicon has 4 valence electrons.
-
These electrons participate in covalent bonding with neighboring atoms in the crystal lattice.
-
The valence band in semiconductor physics refers to the energy band that holds these electrons.
-
❌ Valance (Incorrect in this context)
-
Definition: A decorative drapery hung over a window (totally unrelated to physics or semiconductors).
-
Not used in semiconductor or materials science.
Key Semiconductor Terms Related to Valence:
-
Valence Electrons: Determine bonding properties and conductivity.
-
Valence Band: The highest range of electron energies in which electrons are normally present at absolute zero.