Cadmium Sulfide Wafer (CdS)
CdS Wafers
 We have a large selection of CdS Wafers. If you don't see the specs that you want, please email us your specs and quantity.
We have a large selection of CdS Wafers. If you don't see the specs that you want, please email us your specs and quantity.
Get Your Quote FAST!
Nanopillars' Make Good Photovoltaics
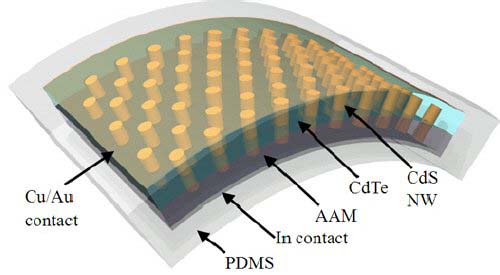
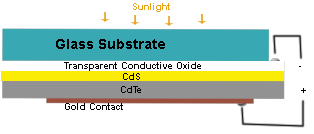
What is Cadmium Sulfide Wafers?
 If you're wondering what is cadmium sulfide, this article will help you. This chemical compound is a solid yellow metal with the chemical formula CdS. It is found naturally in two polymorphs, hexagonal greenockite and chalcogenide. This metal is one of the most common sources of adobe cadmium. It is used in various applications such as pigments, as it has good thermal stability, chemical resistance, and opacity.
If you're wondering what is cadmium sulfide, this article will help you. This chemical compound is a solid yellow metal with the chemical formula CdS. It is found naturally in two polymorphs, hexagonal greenockite and chalcogenide. This metal is one of the most common sources of adobe cadmium. It is used in various applications such as pigments, as it has good thermal stability, chemical resistance, and opacity.
It is an inorganic compound with the chemical formula CdS. It exists naturally in a mixture of zinc ores and rare minerals. It is also present as an impurity substitute in zinc ores. In commercial applications, cadmium sulfide is a primary source of cadmium. It is relatively easy to isolate because it is yellow, and was first used in yellow paint in the 18th century.
What is cadmium sulfide? CdS is a semiconductor material with a low reactivity. This material can be used in solar cells, as well as in a wide range of other applications. It was also one of the first semiconductor materials to be developed for TFTs. After the emergence of amorphous silicon technology in the late 1970s, interest in compound semiconductors for TFTs waned. However, CdS thin films are piezoelectric and are also used as transducers. They have been shown to operate in the GHz region.
In addition to its solar cell uses, CdS is a useful material for TFTs. It was also one of the first compound semiconductors for TFTs, although this technology has recently come on the scene. It is possible to develop a piezoelectric material using a CdS thin film. Its ability to conduct electricity has made it a popular candidate for use in other areas, including telecommunications.
Safety regulations for this substance and its mixtures vary from country to country. Its components are listed in U.S. and Canadian domestic substances lists. In addition to its environmental effects, cadmium sulfide wafers can be contaminated by lead, mercury, or other chemicals. They are not allowed in many environments. A product's safety rating depends on how much it contains of the ingredient.
There are two types of cadmium sulfide wafers. The former is the main form of the substance. It is a solid yellow metal. It is found naturally in zinc ores. It is used for industrial purposes and is also used in paints. The most common form is acetate. It is not a compound of cadmium. The latter is a compound of cadmium and sulfide.
A cadmium sulfide wand is a thin film of cadmium sulfide. The substance is an inorganic compound with the chemical formula CdS. It is found naturally in rare minerals. It is also found in zinc ores. Unlike other inorganic compounds, cadmium sulfide is an inorganic compound with two different crystal structures. The yellow-colored material has many commercial applications.
The safety of cadmium sulfide wands is highly regulated. Its components are listed in the U.S. Environmental Protection Agency's Chemical Substance Inventory and the Canadian Domestic Substances List, but the chemical is safe for use. A cadmium sulfide single crystal wafer ultrasonic washframe can be made from several different materials, but a cadmium sulfide wafer is the simplest to manufacture.
The cadmium sulfide single crystal wand is made by combining cadmium chloride with other layers. It was one of the first materials to be used for TFTs, but interest in compound semiconductors for TFTs declined after the emergence of amorphous silicon technology in the late 1970s. Some thin films of CdS are piezoelectric.
The cadmium sulfide single crystal compound wafer is also known as oxo-cadmium sulfide. This compound is deposited with reactive sputtering. This chemical compound contains oxygen, but it is not necessary. The process is beneficial for both silicon and cadmium solar cells. The oxygen content is measured with Rutherford backscattering spectrometry.
CdS (0001) 5 mm X 5 mm x 0.5 mm , 1 Sides polished
CdS (0001) 5 mm X 5 mm x 0.5 mm , 2 Sides polished
CdS (0001) 5 mm X 5 mm x x1.0 mm, Low resistivities: <1 ohm-cm, 2 sides polished (60/40)
CdS Random orientation 10 mm X 10 mm x 1.0 mm , 2 Sides polished
CdS Random orientation 5 mm X 5 mm x 1.0 mm , 2 Sides polished
CdS (10-10) 10x10x1.0 mm, 2 side polished
CdS (0001) 10x10x0.3 mm, Low resistivities: < 1 ohm-cm, 2 sides polished (2SP)
CdS (0001) 10x10x0.5 mm, 1 side polished (1SP) R:10^3-10^5 ohm.cm
CdS (0001) 10x10x0.5 mm, 2 side polished (2SP)
CdS (0001) co-doped with Li and In, 10x10x1.0 mm, Low resistivities: <1 ohm-cm, 2 sides polished (60/40)
CdS (0001) In doped, 10x10x1.0 mm, Low resistivities: <1 ohm-cm, 2 sides polished (60/40)
CdS (0001) Li doped, 10x10x1.0 mm, Low resistivities: <1 ohm-cm, 2 sides polished (60/40)
CdS (0001) N type, , 10x10x1.0 mm, High resistivities: >1E6ohm-cm, 2 sides polished
CdS (0001) N type, , 10x10x1.0 mm, Low resistivities: <1 ohm-cm, 2 sides polished (60/40)
CdS (0001) N type, , 25.4 mm in dia x1.0 mm, High resistivities: >1E6ohm-cm, 2 sides polished (60/40)
CdS (0001) N type, , 38.1 mm in dia x1.0 mm, Low resistivities: <1 ohm-cm, 2 sides polished (60/40)
